Overview
The article titled "10 Benefits of Partnering with Contract Pharmaceutical Companies" explores the significant advantages that businesses can derive from collaborating with contract pharmaceutical firms. It outlines key benefits including:
- Cost management
- Regulatory compliance
- Quality control
- Enhanced operational efficiency
These partnerships can lead to remarkable improvements in production capabilities and market responsiveness, underscoring the value of such collaborations in today's dynamic industry landscape.
Introduction
In an increasingly competitive pharmaceutical landscape, the decision to partner with contract pharmaceutical companies has evolved into a strategic necessity rather than a mere option. These collaborations present a multitude of advantages, including:
- Cost savings
- Regulatory compliance
- Enhanced quality control
- Improved market agility
Yet, a critical question persists: how can businesses effectively leverage these partnerships to not only survive but thrive in a rapidly evolving industry? This exploration delves into ten key benefits of engaging with contract pharmaceutical manufacturers, offering insights into how these alliances can significantly transform operational efficiency and drive innovation.
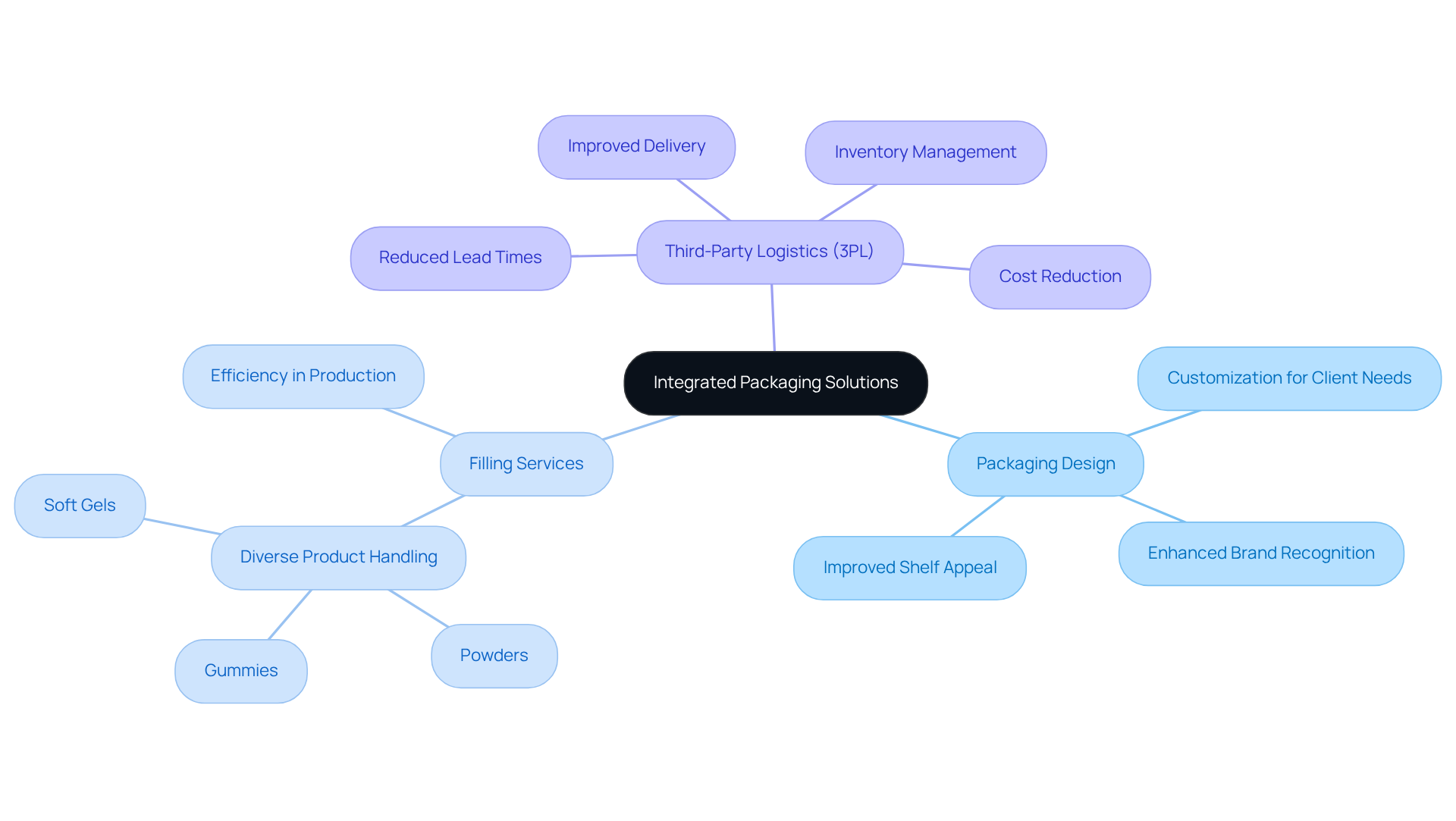
Western Packaging: Integrated Packaging Solutions for Enhanced Supply Chain Efficiency
Western Packaging offers a distinctive integrated approach that combines packaging design, filling services, and third-party logistics (3PL) into a cohesive service model. This strategy streamlines the supply chain and significantly enhances operational efficiency. By managing every aspect of packaging and distribution, businesses can effectively reduce lead times and improve product delivery. This comprehensive management not only results in enhanced customer satisfaction and loyalty but also allows businesses to focus on their core competencies. They benefit from customized solutions that address specific packaging needs. Industry leaders assert that integrated packaging solutions are essential for driving efficiency and sustainability in today’s competitive market, ultimately transforming how manufacturers operate and engage with their customers.
Cost Management: Reducing Expenses Through Contract Pharmaceutical Partnerships
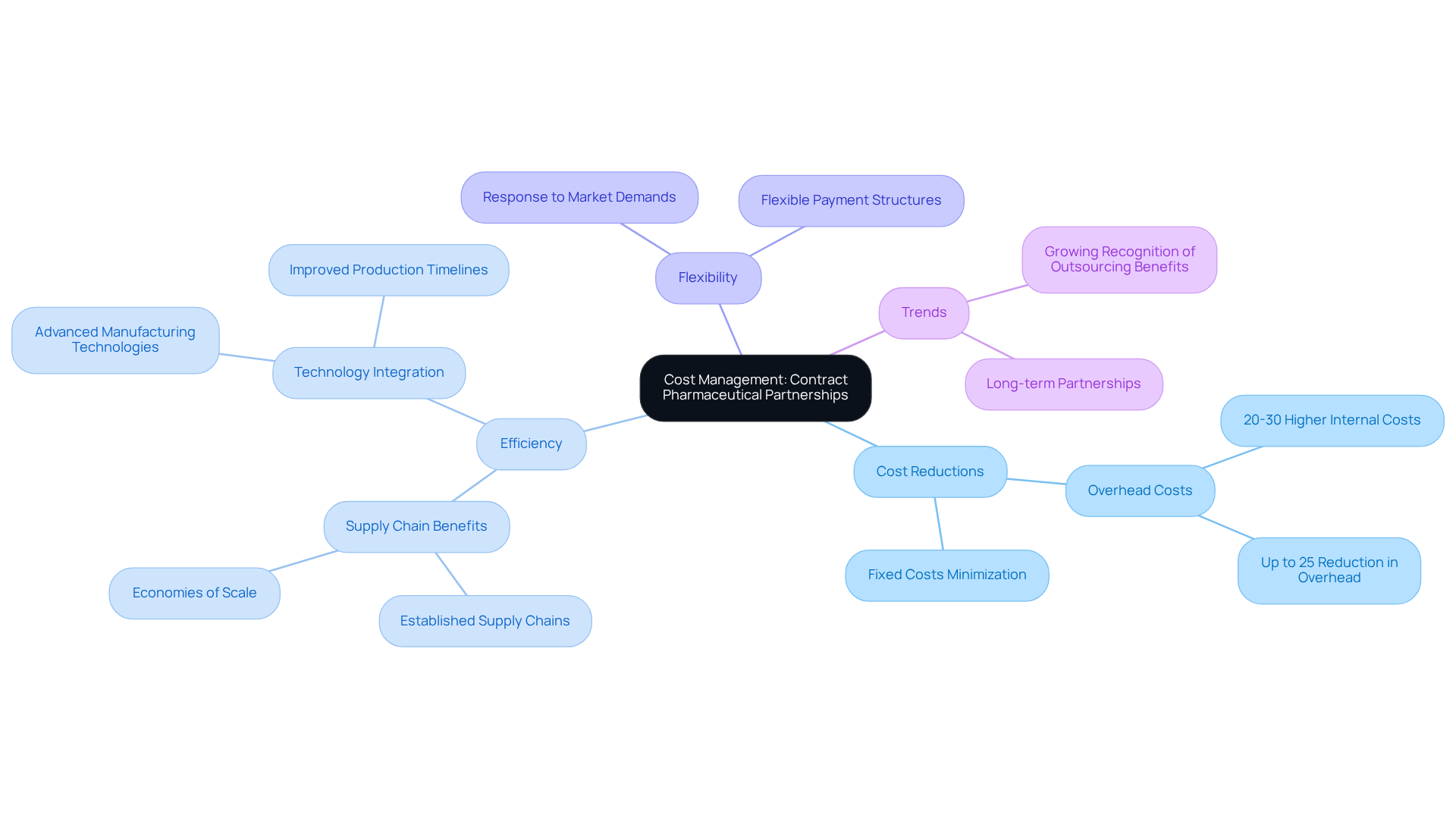
Collaborating with contract pharmaceutical companies can lead to significant cost reductions by effectively managing overhead costs. By outsourcing production, companies can circumvent the substantial overhead expenses tied to maintaining internal manufacturing facilities, which can be 20-30% higher than those associated with external manufacturing. Contract manufacturers capitalize on established supply chains and economies of scale, allowing them to produce products more efficiently and at a lower cost. This efficiency results in reduced expenses for clients, enabling them to allocate resources more strategically.
Financial analysts emphasize that outsourcing production not only minimizes fixed costs but also enhances flexibility in responding to market demands. Firms that have transitioned to outsourced manufacturing report overhead decreases of up to 25%, freeing up capital for innovation and growth initiatives. Furthermore, the integration of advanced technologies and efficient processes by manufacturers can lead to improved production timelines and quality, further driving down expenses.
Recent trends suggest that businesses are increasingly acknowledging the economic advantages of outsourcing. As the pharmaceutical sector evolves, numerous firms are embracing flexible payment structures with contract pharmaceutical companies, which helps align financial interests while mitigating risks associated with production. This transition not only supports cost management but also nurtures long-term partnerships that can adapt to shifting market conditions, ultimately enhancing operational efficiency and competitiveness.
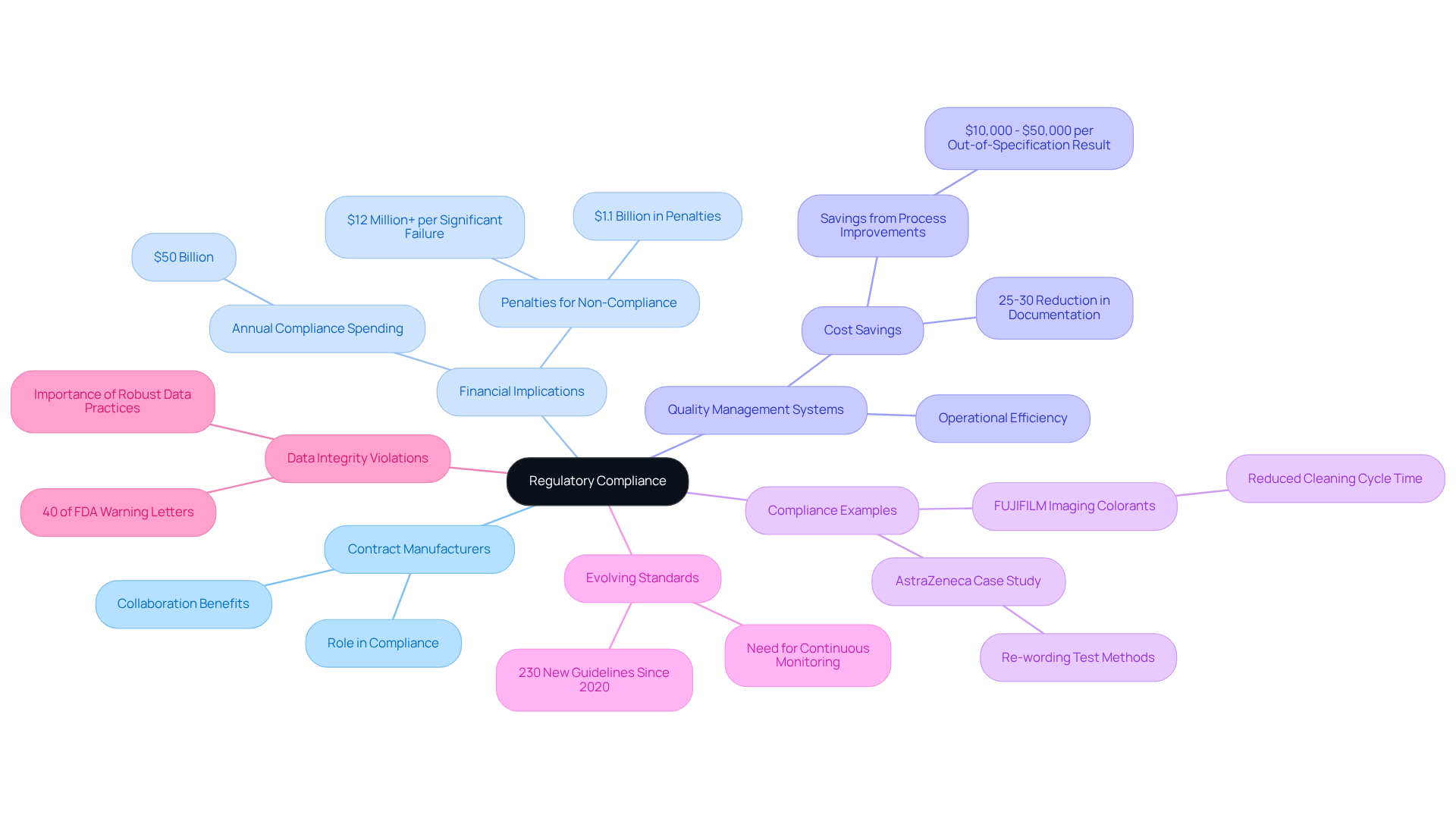
Regulatory Compliance: Ensuring Adherence to Industry Standards with Contract Manufacturers
Contract pharmaceutical companies play a pivotal role in navigating the intricate regulatory landscape, ensuring that all products comply with stringent industry standards. By collaborating with these specialists, businesses can effectively mitigate the risks associated with non-compliance, which can lead to significant penalties and reputational damage. For instance, companies that implement modern Quality Management Systems (QMS) report a 25-30% reduction in documentation efforts, streamlining compliance processes and enhancing operational efficiency. Notably, the pharmaceutical industry spends approximately $50 billion annually on global regulatory compliance, underscoring the financial implications of maintaining adherence to these standards.
A notable example is AstraZeneca, which transformed its quality assurance processes by re-wording test methods, saving between $10,000 and $50,000 for each out-of-specification result prevented. This proactive strategy not only protects product integrity but also enhances brand reputation in a competitive environment. Furthermore, compliance failures have resulted in $1.1 billion in penalties over the past five years, highlighting the critical need for effective compliance strategies.
As of 2025, the pharmaceutical industry continues to face evolving standards, with over 230 new or revised guidelines issued since 2020. This dynamic environment requires collaborations with manufacturing partners who are skilled at ensuring compliance. Regulatory experts emphasize that viewing compliance as a strategic advantage rather than a burden can significantly enhance a company's market position. As one regulatory consultant noted, "Missing a single guidance document can put you months behind competitors." By utilizing the knowledge of contract pharmaceutical companies, businesses can guarantee compliance with industry standards while concentrating on their core strengths, ultimately fostering growth and innovation. Additionally, with 40% of FDA warning letters citing data integrity violations, maintaining robust data practices is essential for compliance. Companies are encouraged to assess their current compliance strategies against these statistics and examples to enhance their operational effectiveness.
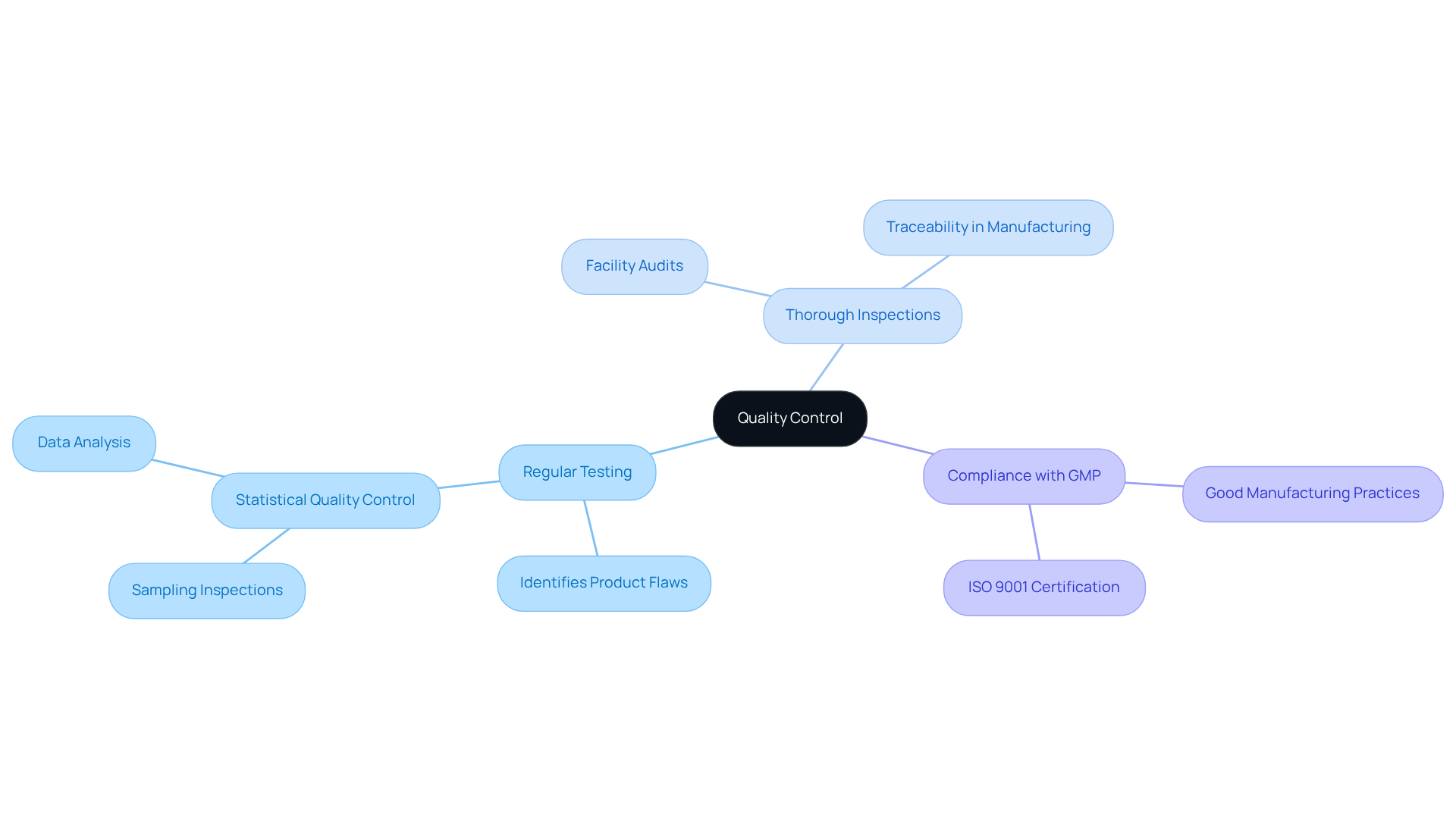
Quality Control: Maintaining High Standards Through Contract Manufacturing
Contract manufacturers enforce stringent quality control measures to guarantee that products adhere to high standards. This encompasses:
- Regular testing
- Thorough inspections
- Strict compliance with Good Manufacturing Practices (GMP)
By capitalizing on the expertise of these manufacturers, companies can ensure consistent product quality—a vital component for fostering consumer trust and loyalty.
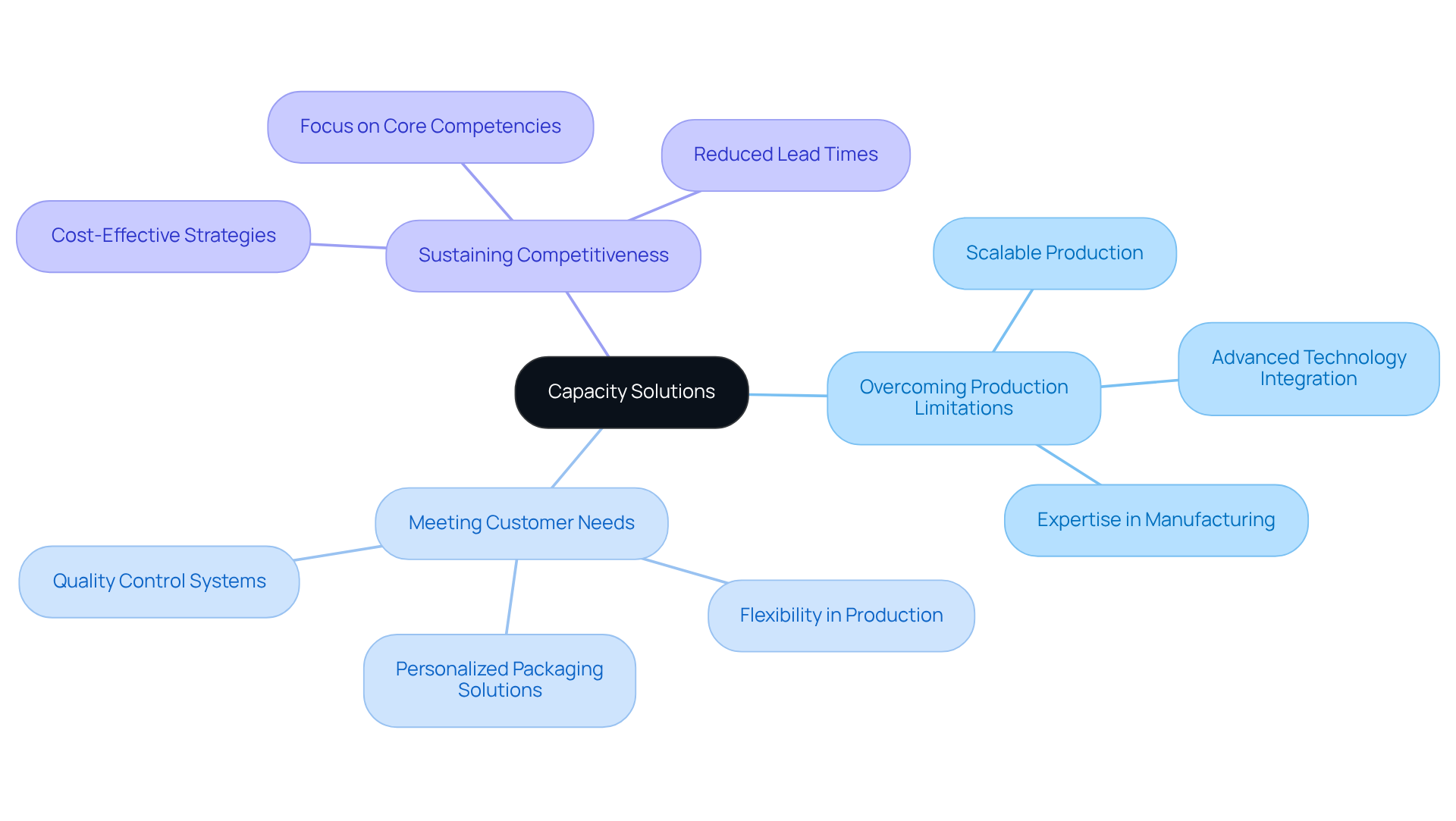
Capacity Solutions: Overcoming Production Limitations with Contract Partnerships
Contract pharmaceutical companies deliver scalable production solutions that adeptly adjust to fluctuating demand conditions.
By collaborating with contract pharmaceutical companies, businesses can effectively overcome production limitations, ensuring they meet customer needs without the burden of investing in additional infrastructure.
This adaptability is not merely beneficial; it is essential for sustaining competitiveness in a rapidly evolving environment.
Speed to Market: Accelerating Product Launches with Contract Pharmaceutical Companies
Collaborating with pharmaceutical firms can significantly accelerate the product development and launch process. These manufacturers harness established processes and extensive resources, resulting in notably quicker turnaround times. The development and manufacturing organization (CDMO) sector is projected to grow from $168 billion in 2025 to $318 billion by 2034, driven by advancements in R&D, biologics, and personalized therapies. This growth underscores the increasing demand for efficient product launches.
Businesses that have integrated manufacturing agreements into their strategies have reported remarkable advancements in their entry schedules. A notable example is a company that enhanced its packaging line production to 60,000 tubes daily within a mere six months, illustrating how rapid scaling can directly improve speed to consumers and effectively meet their demands.
The impact of established processes on product development speed is substantial. By leveraging the capabilities of production firms, companies can optimize their processes, shorten lead times, and enhance their ability to respond swiftly to industry trends and consumer needs. As Dr. Hemant N. Joshi emphasizes, the importance of speed to entry is critical in today's competitive environment, where timely access to innovative therapies can significantly influence patient outcomes. His extensive experience with various drug molecules highlights the challenges faced in the industry, reinforcing the necessity for efficient processes.
In conclusion, collaborating with contract pharmaceutical companies not only expedites product introductions but also empowers organizations to capitalize on emerging opportunities in the industry. This collaboration ultimately leads to improved operational efficiency and a strengthened market presence. However, it remains essential to be mindful of the potential risks associated with expedited pathways, ensuring that safety and efficacy are never compromised.
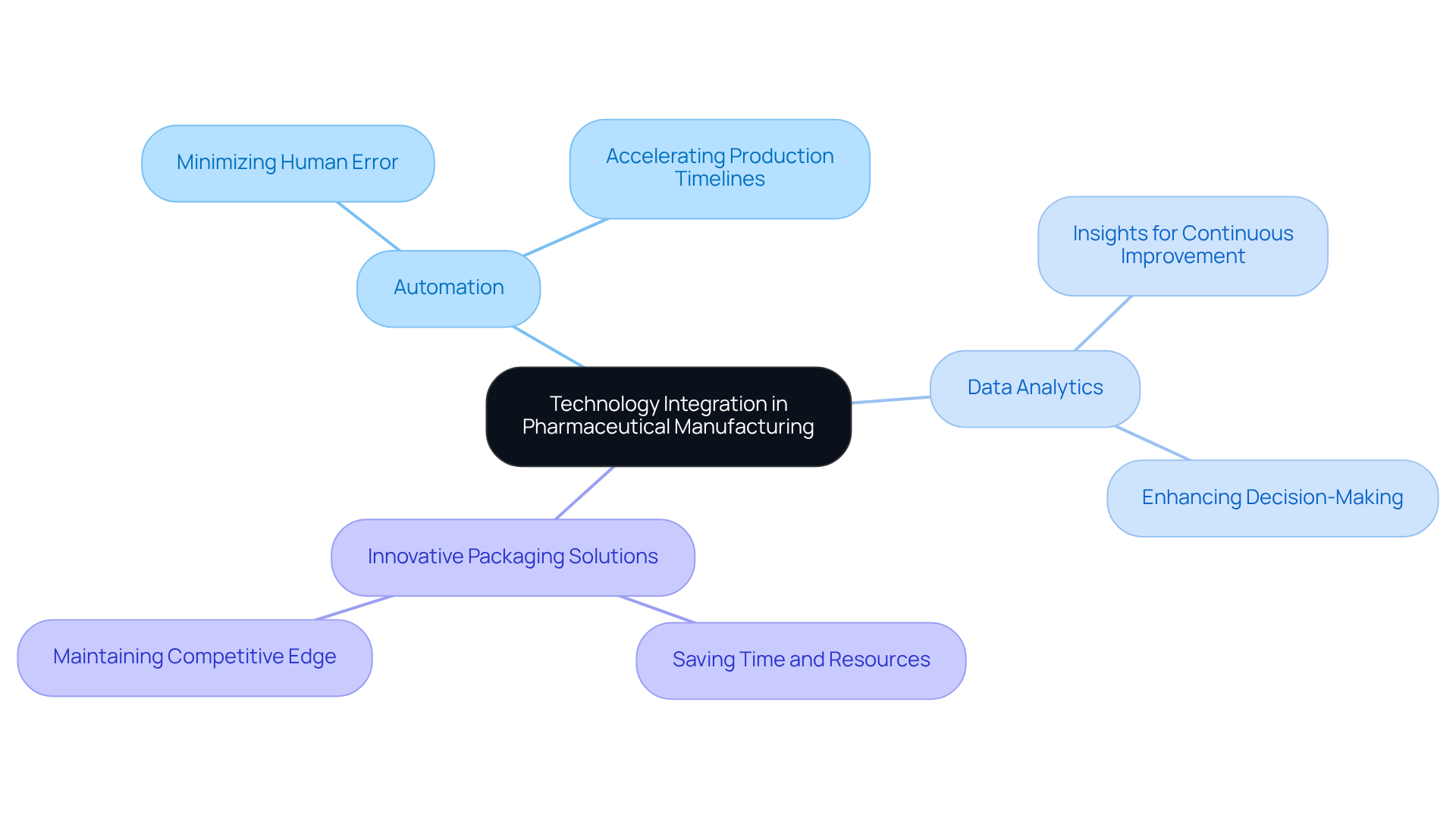
Technology Integration: Leveraging Innovations in Contract Pharmaceutical Manufacturing
Contract pharmaceutical companies stand at the forefront of technological advancements, investing significantly in automation, advanced data analytics, and innovative packaging solutions to optimize their manufacturing processes. These technologies not only streamline operations but also enhance efficiency and product quality considerably.
For example, automation minimizes human error and accelerates production timelines, while data analytics offers valuable insights that drive continuous improvement. As Kofi Annan aptly noted, 'Innovation is the call of the future,' underscoring its critical role in the industry. Moreover, optimizing the packaging process enables clients to save time and resources, which is essential for maintaining a competitive edge.
Consequently, companies that embrace these innovations can expect enhanced performance and a competitive advantage in the sector. Industry leaders consistently emphasize that innovation is vital for success; as Steve Jobs stated, 'Innovation is the ability to see change as an opportunity, not a threat.' This perspective is crucial as organizations navigate the evolving landscape of pharmaceutical manufacturing in 2025 and beyond.
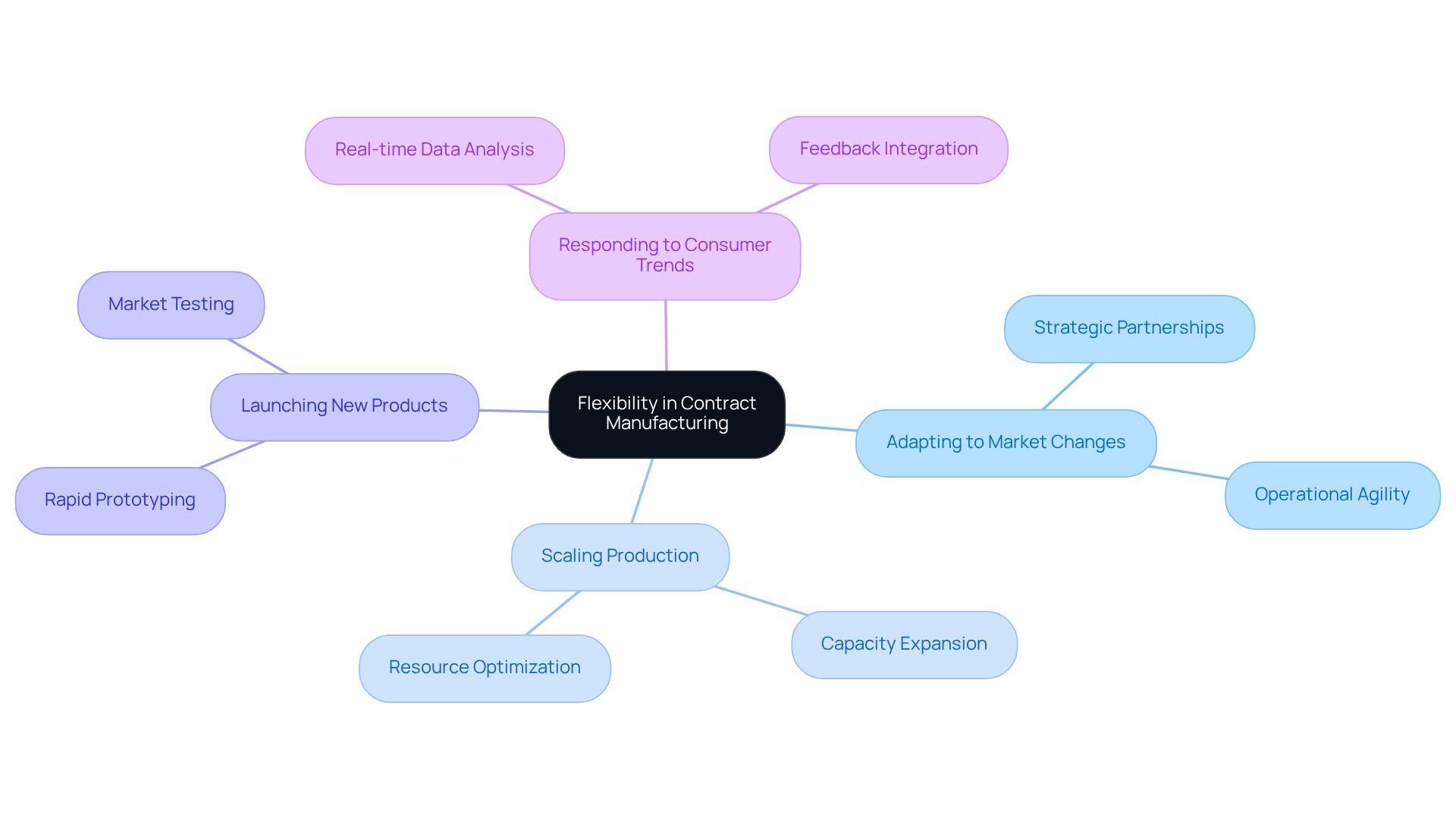
Flexibility: Adapting to Market Changes with Contract Manufacturing Solutions
Contract pharmaceutical companies offer essential manufacturing solutions for companies aiming to adapt to changing economic conditions. These strategic partnerships empower businesses to scale production as needed, launch new product lines, and respond swiftly to emerging consumer trends. The ability to adjust operations is critical for maintaining competitiveness in today’s dynamic environment.
Industry analysts highlight that interconnected and adaptable supply systems depend on visibility and seamless interaction, which are achieved through strategic alliances. For instance, companies that have embraced manufacturing agreements with contract pharmaceutical companies have successfully adjusted their production strategies to meet fluctuating demands, illustrating the significant impact of flexible production on market responsiveness.
As we approach 2025, the importance of scalability in production will only grow, making manufacturing partners invaluable allies in achieving operational agility and sustained growth.
Strategic Partnerships: Building Long-Term Relationships with Contract Manufacturers
Establishing strategic partnerships with contract pharmaceutical companies results in long-term benefits for both parties involved. These connections foster teamwork, ignite creativity, and align common objectives, ultimately enhancing product development and commercial success. By collaborating closely with manufacturers, businesses can synchronize their strategies and ensure that their products effectively meet consumer needs.
A partnership grounded in trust serves as a formidable weapon against competitors, offering a significant competitive edge. Successful collaborations hinge on clearly defined expectations and mutual value creation, which are essential for grasping the foundation of effective partnerships. The biologics market, for instance, is projected to expand at a compound annual growth rate of 15% until 2027, highlighting the necessity for agile partnerships capable of adapting to evolving market conditions.
Business leaders consistently underscore the transformative power of collaboration. As Howard Schultz aptly stated, 'Success is best when it’s shared.' This sentiment resonates profoundly within the pharmaceutical sector, where contract pharmaceutical companies can drive innovation and enhance product offerings.
Moreover, long-term partnerships with manufacturers can result in shorter lead times and improved product delivery, empowering businesses to focus on their core strengths while enhancing operational efficiency. The essence of these partnerships resides in mutual trust and clear communication, which are vital for achieving shared goals.
In summary, the advantages of partnering with manufacturing firms extend beyond short-term profits, fostering a culture of innovation and resilience that is crucial for enduring success in the competitive pharmaceutical landscape.
Comprehensive Services: Streamlining Operations with Contract Pharmaceutical Partnerships
Collaborations with contract pharmaceutical companies that provide integrated services can significantly enhance operations for businesses. By consolidating manufacturing, packaging, and logistics under one umbrella, these partnerships simplify the management of supply chains, thereby reducing the complexity associated with coordinating multiple vendors. This cohesive strategy not only saves valuable time and resources but also significantly improves overall supply chain efficiency, allowing organizations to focus on their core competencies.
The impact of integrated manufacturing and logistics on supply chain complexity is profound. As noted by industry experts, competition increasingly hinges on the effectiveness of supply chains rather than individual companies. The COVID-19 pandemic highlighted critical vulnerabilities in pharmaceutical supply chains, underscoring the need for robust, integrated solutions that can withstand disruptions. In fact, supply chains cannot tolerate even 24 hours of disruption, making streamlined operations essential for maintaining market share.
Many companies have successfully navigated these challenges by partnering with contract pharmaceutical companies. For example, Oragenics' collaboration with Sterling Pharma Solutions for GMP manufacturing exemplifies how such partnerships can enhance manufacturing capabilities while ensuring compliance with regulatory standards. This collaboration not only aids the creation of pharmaceutical products but also enables organizations to concentrate on drug discovery and marketing, thus enhancing operational efficiency.
Experts such as Tom Peters stress that logistics is a key factor in achieving success, stating that 'leaders win through superior logistics.' This highlights the critical role that integrated services play in enhancing operational effectiveness. As the industry evolves, the importance of digitization in enhancing transportation and logistics services becomes increasingly clear, as noted by Søren Skou. By leveraging integrated services, pharmaceutical companies can achieve significant cost savings, scalability, and regulatory compliance, ultimately facilitating faster market entry for new drugs. In 2025, streamlining operations with contract pharmaceutical companies will be more critical than ever, as businesses seek to optimize their supply chains and enhance their competitive edge.
Conclusion
Partnering with contract pharmaceutical companies offers a multitude of advantages that can significantly enhance operational efficiency and competitiveness within the pharmaceutical sector. These collaborations not only streamline production processes but also foster innovation, regulatory compliance, and quality assurance, ultimately positioning businesses for long-term success in a rapidly evolving market.
Throughout this article, key benefits such as cost management, capacity solutions, speed to market, and technology integration have been thoroughly explored. Businesses that leverage these partnerships can reduce expenses, adapt to changing market demands, and accelerate product launches while ensuring adherence to stringent regulatory standards. The emphasis on strategic partnerships underscores the importance of collaboration in achieving shared goals and cultivating a culture of innovation, which is essential for thriving in a competitive landscape.
As the pharmaceutical industry continues to evolve, embracing the benefits of contract manufacturing will be crucial for organizations seeking to maintain a competitive edge. Companies are encouraged to assess their current strategies and consider the transformative potential of these partnerships. By doing so, they can not only enhance operational efficiency but also position themselves to capitalize on emerging opportunities, driving growth and success in the years to come.
Frequently Asked Questions
What is the integrated approach offered by Western Packaging?
Western Packaging provides an integrated approach that combines packaging design, filling services, and third-party logistics (3PL) into a cohesive service model, streamlining the supply chain and enhancing operational efficiency.
How does Western Packaging's integrated approach benefit businesses?
This approach reduces lead times, improves product delivery, enhances customer satisfaction and loyalty, and allows businesses to focus on their core competencies while receiving customized packaging solutions.
What are the cost management benefits of collaborating with contract pharmaceutical companies?
Collaborating with contract pharmaceutical companies can lead to significant cost reductions by managing overhead costs, as outsourcing production can be 20-30% cheaper than maintaining internal manufacturing facilities.
How does outsourcing production enhance flexibility for companies?
Outsourcing production minimizes fixed costs and allows firms to respond more flexibly to market demands, with companies reporting overhead decreases of up to 25%, freeing up capital for innovation and growth.
What role do contract manufacturers play in regulatory compliance?
Contract pharmaceutical companies help navigate the regulatory landscape, ensuring products comply with industry standards and mitigating risks associated with non-compliance, which can lead to penalties and reputational damage.
What financial implications does regulatory compliance have for the pharmaceutical industry?
The pharmaceutical industry spends approximately $50 billion annually on global regulatory compliance, highlighting the significant costs associated with maintaining adherence to standards.
How can companies enhance their compliance strategies?
Companies can enhance their compliance strategies by collaborating with contract pharmaceutical companies, utilizing modern Quality Management Systems (QMS), and viewing compliance as a strategic advantage to improve market position.
What are the consequences of compliance failures in the pharmaceutical industry?
Compliance failures have resulted in $1.1 billion in penalties over the past five years, underscoring the critical importance of effective compliance strategies to protect product integrity and brand reputation.




