Overview
Serialization in the pharmaceutical sector for nutraceuticals offers key benefits that cannot be overlooked. Enhanced product traceability, improved compliance with regulatory requirements, and increased consumer trust are paramount, particularly in preventing counterfeit products. Serialization systems facilitate tracking throughout the supply chain, which is crucial for manufacturers striving to comply with laws such as the Drug Supply Chain Security Act. This compliance not only safeguards consumer safety but also reinforces brand integrity, establishing a foundation of reliability that consumers can trust.
Introduction
Serialization has emerged as a critical component in the pharmaceutical industry, particularly within the nutraceutical sector, where the integrity of products is paramount. By implementing serialization, companies can enhance traceability and compliance, while simultaneously combating the pervasive threat of counterfeit drugs that jeopardize consumer safety.
As the landscape evolves, manufacturers face a pressing question: how can they effectively navigate the complexities of serialization while maximizing its benefits in an increasingly competitive market?
This article delves into the key advantages of serialization in pharma, exploring its transformative impact on operations, regulatory compliance, and consumer trust.
Western Packaging: Integrated Serialization Solutions for Pharma
Western Packaging delivers cohesive tracking solutions that significantly enhance the packaging and distribution processes for pharmaceutical items, particularly within the nutraceutical sector. By leveraging their expertise in packaging design, filling services, and comprehensive third-party logistics (3PL) services—including warehousing, inventory management, and distribution—they facilitate the seamless integration of product tracking into the supply chain. This all-encompassing strategy not only enhances item traceability but also fortifies brand integrity, empowering manufacturers to effectively meet industry regulations and customer expectations.
The implementation of data encoding markedly reduces the risk of counterfeit products infiltrating the market, thereby safeguarding consumer safety and fostering trust in brands. Furthermore, as data formatting evolves into a regulatory requirement across more than 100 nations, Western Packaging's proactive approach ensures that clients remain compliant while benefiting from improved inventory management and operational efficiency.
Strategic planning, supported by their extensive logistics solutions, is vital for the success of tracking projects, enabling producers to navigate the complexities involved. As the industry progresses, staying ahead of serialisation pharma trends becomes essential for manufacturers seeking to bolster their market presence and streamline their supply chains.
![]()
What is Serialization in Pharma? Understanding the Basics
The process of serialisation pharma in the pharmaceutical sector is critical as it involves assigning a unique identifier to each saleable unit of an item, typically represented by a combination of numbers and letters. This identifier can be scanned and monitored throughout the supply chain, playing a crucial role in ensuring authenticity. By enabling manufacturers to track their goods from production to point-of-sale, item tracking significantly enhances transparency and accountability within the industry.
The significance of data sequencing is underscored by regulatory requirements that compel pharmaceutical firms to invest in serialisation pharma systems. Such systems are essential for maintaining compliance and protecting integrity, particularly for smaller distributors who may struggle to adopt these advanced technologies. Case studies reveal that major players in the pharmaceutical industry have successfully implemented serialisation pharma systems, while smaller entities often lag behind, adversely impacting their operating income.
Unique identifiers not only facilitate product tracking but also play a pivotal role in combating counterfeit drugs, a substantial threat to patient safety. Serialisation pharma enables the unique identification of drugs, making replication difficult and ensuring that only authorized trading partners participate in the distribution process. This capability is vital for preserving the integrity of the supply chain and safeguarding buyers, as illustrated in the case study 'Combating Counterfeit Drugs through serialisation pharma practices.'
Real-world examples demonstrate that businesses implementing tracking have reported increased operational efficiency and a strengthened market presence, as they can better manage inventory and respond to regulatory demands. Moreover, distributors must possess the necessary equipment for scanning serial numbers and comprehensive data collection systems to effectively meet regulatory requirements. The extensive investment in serialisation pharma transcends mere compliance; it represents a strategic initiative that enhances overall supply chain security and fosters trust among customers.
![]()
Counterfeit Prevention: The Role of Serialization in Pharma
Serialization pharma plays a pivotal role in the fight against counterfeit nutraceuticals, providing each product with a unique identifier that enables producers to verify authenticity across the supply chain. This critical process not only shields consumers from potentially harmful counterfeit products but also strengthens brand reputation, fostering consumer trust in the products they purchase. The World Health Organization estimates that 4 out of 10 medications in developing nations may be contaminated, leading to significant financial losses for pharmaceutical companies due to fake drugs. This alarming statistic underscores the urgent need for robust encoding measures. By implementing data encoding, manufacturers can significantly reduce the risk of counterfeit items entering the market, as it facilitates precise monitoring and tracing of each batch.
For example, the United States Drug Supply Chain Security Act (DSCSA) has established a comprehensive framework for identifying and tracing prescription drugs, which has effectively diminished counterfeit incidents. This legislation not only applies to pharmaceuticals but also sets a crucial precedent for nutraceuticals, highlighting the necessity of serialisation pharma and traceability across all sectors. In 2019, there were 239 convictions related to counterfeit pharmaceuticals, which illustrates the ongoing challenges in this domain and demonstrates the efficacy of tracking in addressing these issues. Industry leaders assert that serialisation pharma transcends mere compliance; it enhances operational efficiency and provides essential insights into product authenticity. As one expert noted, 'The most essential aim of tracking is to gain insight into intricate supply chains throughout the production, shipping, and distribution activities of pharmaceutical firms.'
Successful examples of data encoding in practice include Turkey's Pharmaceutical Track and Trace System, which has notably improved oversight of pharmaceuticals and reduced counterfeit occurrences by over 30% since its inception. By adopting advanced packaging technologies, nutraceutical producers not only protect their clients but also cultivate a trustworthy brand reputation, ultimately leading to increased market trust and customer loyalty. To effectively implement data encoding, producers should consider investing in robust tracking systems and collaborating with regulatory organizations to ensure compliance and enhance consumer confidence.
Regulatory Compliance: Serialization Requirements for Pharma Manufacturers
Pharmaceutical producers are required to comply with various tracking regulations, notably the Drug Supply Chain Security Act (DSCSA) in the United States. These regulations necessitate that manufacturers adopt serialisation pharma practices to bolster traceability and accountability. Compliance not only mitigates the risk of legal repercussions but also fosters consumer confidence in the safety and authenticity of pharmaceutical products.
Supply Chain Efficiency: How Serialization Improves Operations
Serialisation pharma is pivotal in enhancing supply chain efficiency through real-time monitoring of goods. This capability empowers manufacturers to swiftly identify and address issues such as stock discrepancies and recalls, ultimately minimizing disruptions. The integration of data formatting not only streamlines operations but also significantly reduces lead times, enabling businesses to respond more adeptly to market demands.
For instance, organized data has proven effective in uncovering diversion schemes, leading to substantial revenue recovery. Moreover, logistics experts assert that real-time tracking elevates service levels and operational efficiency, which in turn boosts customer satisfaction.
By leveraging data formatting alongside Western Packaging's integrated filling services—capable of handling a variety of items from powders to gummies—and extensive 3PL solutions, nutraceutical producers can significantly enhance their supply chains with serialisation pharma. This strategic approach ensures timely and accurate product delivery, a critical factor in a competitive market.
Implementation Challenges: Navigating Serialization in Pharma
Introducing data encoding for serialisation pharma presents significant challenges for pharmaceutical producers, primarily due to the substantial investment required in technology and training. The integration of data encoding into existing systems can be complex, demanding meticulous planning and execution.
Furthermore, manufacturers must ensure their workforce is sufficiently trained to navigate new processes and technologies, a task that can be both time-consuming and resource-intensive.
It is essential for companies to prioritize serialisation pharma to maintain operational efficiency and compliance in an increasingly data-driven environment.
Technological Innovations: Advancements in Serialization Solutions
Recent advancements in technology have revolutionized data arrangement methods, particularly in serialisation pharma within the pharmaceutical industry. Innovations such as blockchain technology and IoT devices, combined with advanced data analytics, significantly enhance traceability and security. Blockchain, with its immutability and decentralized nature, provides a robust framework for managing serialized data in serialisation pharma, ensuring that every transaction is recorded transparently and securely. This technology not only simplifies the tracking process but also mitigates risks associated with counterfeit items—a critical concern, given that nearly half a million individuals in sub-Saharan Africa die each year due to counterfeit medicines, including 267,000 fatalities linked to falsified or low-quality antimalarial medications.
IoT devices further amplify these capabilities by facilitating real-time tracking and monitoring of goods throughout the supply chain. For instance, RFID tags, which are projected to witness the fastest growth in the tracking market, enable quicker and more precise scanning, thereby enhancing inventory management and reducing the likelihood of errors. The RFID tags segment is anticipated to expand rapidly due to their superior tracking capabilities and improved supply chain efficiency.
The integration of these technologies provides producers with invaluable insights into consumer behavior and performance, enabling more informed decision-making. As over 75% of global medicines were subject to some form of track and trace regulations by 2019, the adoption of blockchain and IoT solutions is increasingly essential for companies seeking to enhance compliance and operational efficiency. By leveraging these innovations, pharmaceutical producers can not only improve their serialisation pharma processes but also strengthen their market position in an increasingly competitive landscape.
![]()
Product Recalls: The Impact of Serialization on Efficiency
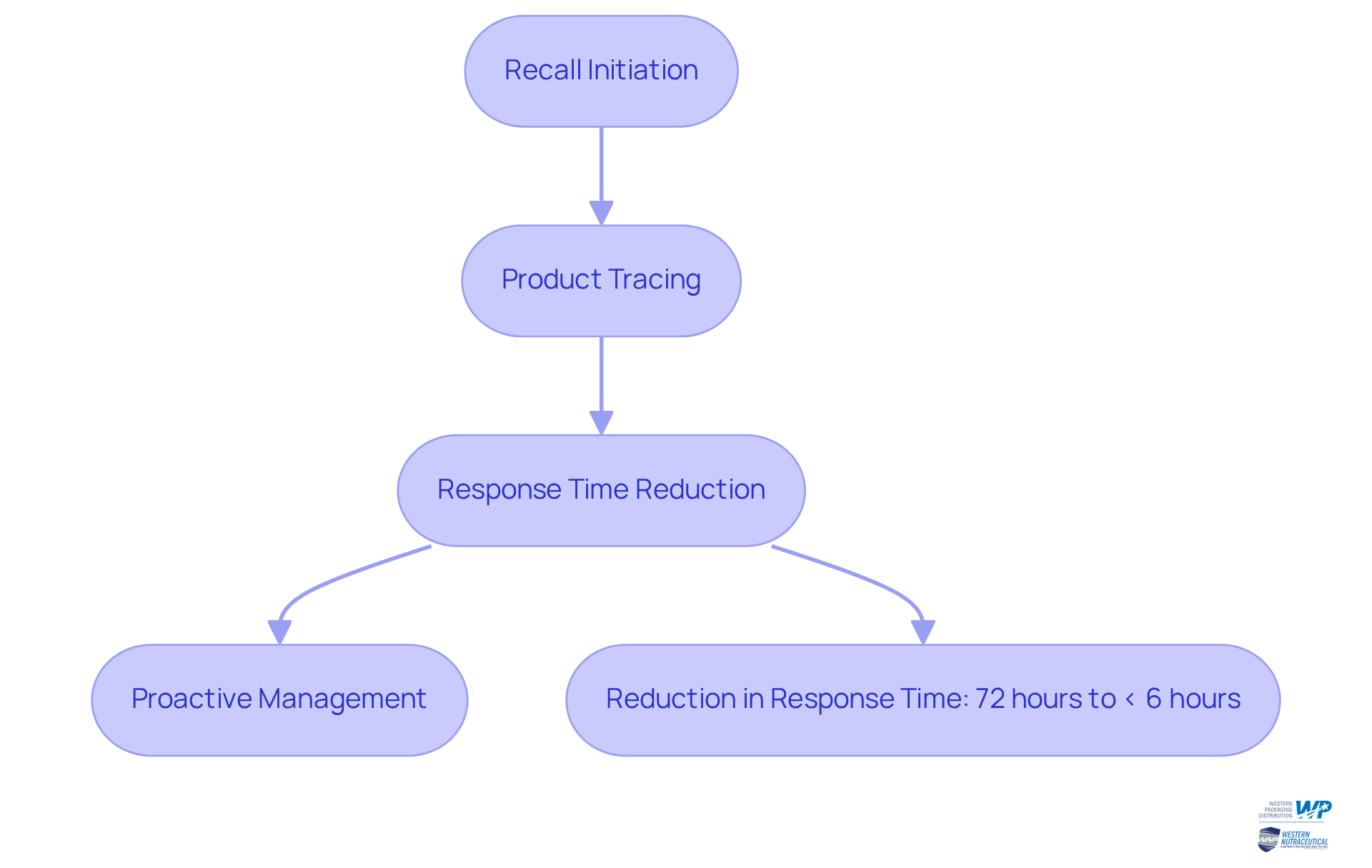
Serialisation pharma significantly enhances the efficiency of recalls by enabling manufacturers to swiftly identify and locate affected items. When a recall is initiated, serialisation pharma enables precise tracing of products throughout the supply chain, drastically reducing the time required to withdraw potentially harmful products from circulation.
The execution of data packaging has been shown to reduce recall response durations from an average of 72 hours to less than 6 hours, as illustrated by Pharma Trax, affecting only a minor portion of distributed units. This rapid response not only safeguards consumers but also preserves brand integrity, as companies can act decisively to mitigate risks.
Effective coding transforms recall management from a reactive process into a proactive, data-driven strategy crucial for serialisation pharma. As highlighted by Pharma Trax, "When applied strategically, product tracing transforms recall response from reactive firefighting to a proactive, data-driven process," enhancing overall operational efficiency.
By utilizing data encoding, nutraceutical producers can guarantee compliance with regulatory standards while managing recalls with agility and precision, ultimately fostering greater trust among consumers. The dangers linked to poor recall management emphasize the essential requirement for strong tracking methods.
Data Management: Key to Successful Serialization in Pharma
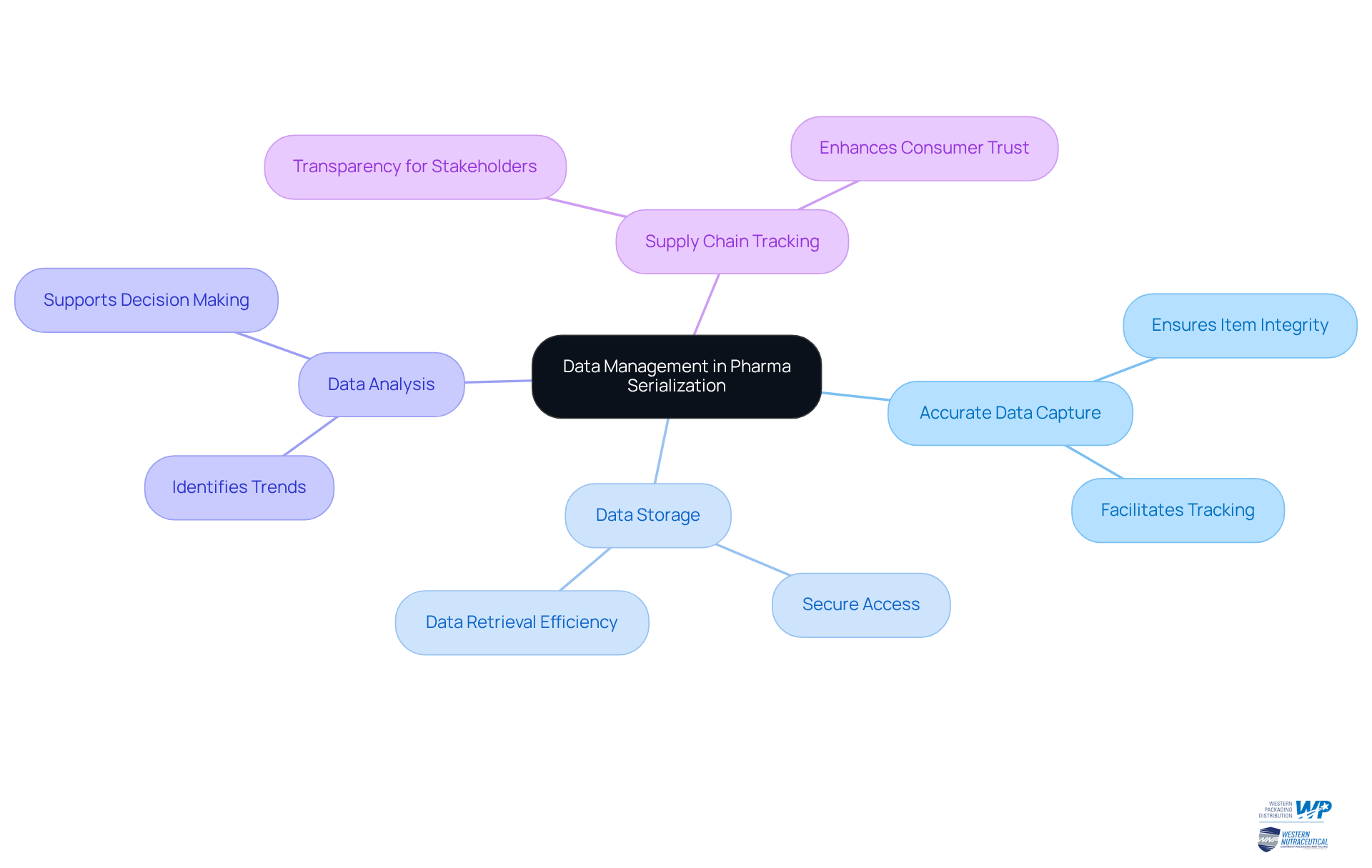
Successful serialization pharma hinges on effective data management practices. Manufacturers must ensure that serialized data related to serialisation pharma is accurately captured, stored, and analyzed to uphold item integrity. By implementing robust data management systems, manufacturers can ensure serialisation pharma, allowing them to meticulously track products throughout the supply chain and guaranteeing that all stakeholders have access to accurate and current information. This level of transparency is not merely beneficial; it is essential for compliance and fostering consumer trust.
Future Trends: The Evolution of Serialization in the Pharmaceutical Industry
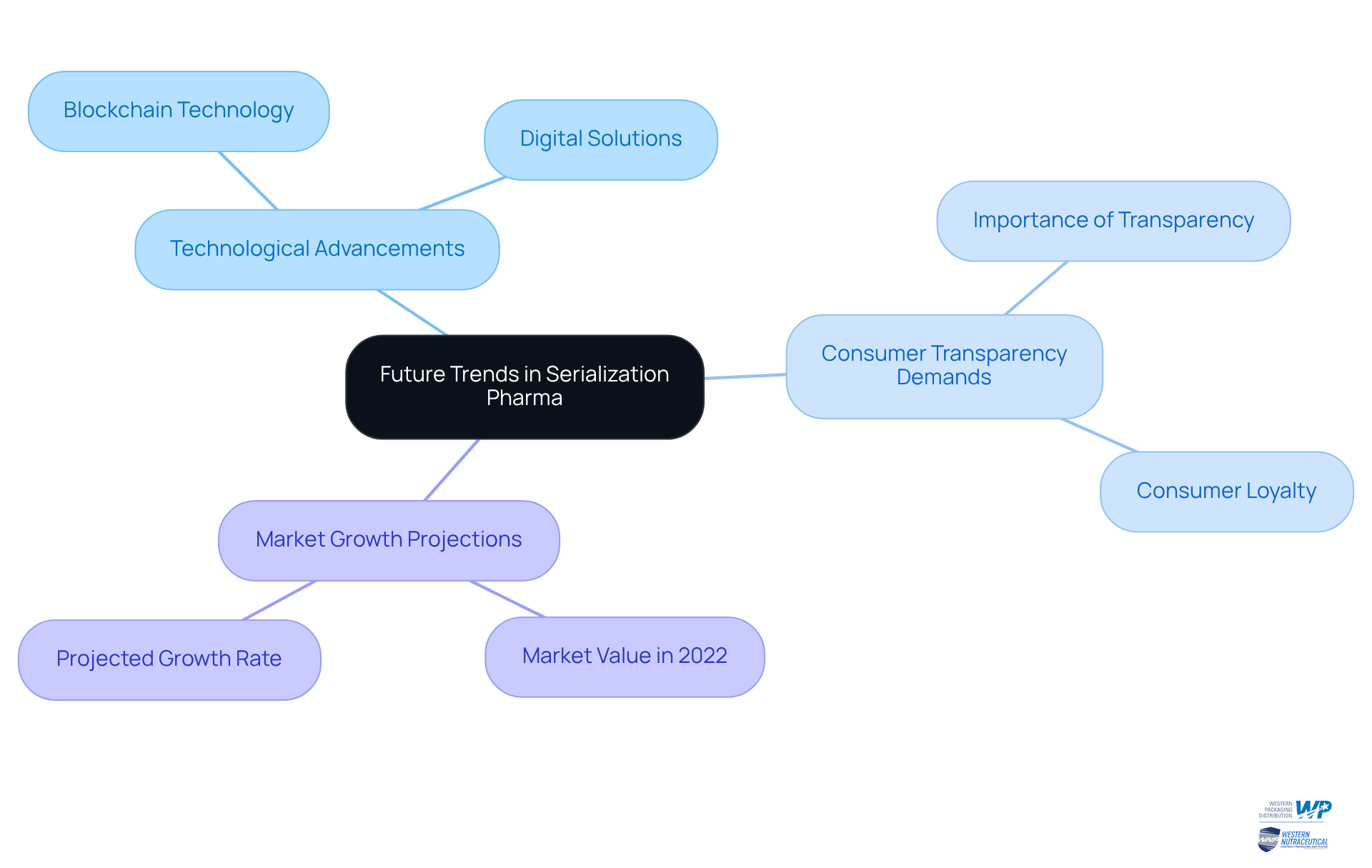
The future of data encoding in the pharmaceutical sector, particularly in serialisation pharma, is poised for substantial transformation, driven by rapid technological advancements and evolving regulatory frameworks. As manufacturers increasingly embrace digital solutions, we anticipate a seamless integration of product tracking within broader supply chain operations. This shift transcends mere operational changes; it reflects a growing public demand for transparency and product authenticity. Research indicates that 73% of consumers consider transparency essential when selecting brands, underscoring the necessity for producers to adapt their tracking methodologies accordingly. Moreover, studies reveal a statistically significant positive correlation between brands that achieve higher transparency scores and increased sales growth, emphasizing the critical role of transparency in fostering consumer loyalty.
Additionally, the pharmaceutical tracking market was valued at US$ 15.1 billion in 2022 and is projected to grow at a compound annual growth rate (CAGR) of 5.1% from 2023 to 2031, exceeding US$ 24.8 billion by 2031. This growth underscores the urgency for companies to innovate. Industry leaders assert that effective tracking methods are vital for compliance with stringent regulations and for combating the escalating threat of counterfeit medications. For instance, companies like Roche and Pfizer are making significant investments in advanced tracking technologies, including the integration of blockchain technology, to enhance item traceability and ensure regulatory compliance.
Advancements in serialisation pharma methods, including the application of blockchain technology, provide a high level of transparency and security in monitoring pharmaceutical products. This integration not only improves supply chain visibility but also cultivates consumer trust, as brands that prioritize transparency are more likely to foster loyalty among their customers. As the landscape continues to evolve, manufacturers must remain agile, leveraging digital solutions to meet the rising expectations for product integrity and safety.
Conclusion
The significance of serialization in the pharmaceutical industry, particularly for nutraceuticals, is paramount. This strategic approach not only enhances product traceability and combats counterfeit threats but also ensures compliance with evolving regulatory standards. By adopting serialization practices, manufacturers can bolster their operational efficiency and maintain consumer trust, which is increasingly vital in today's market landscape.
Throughout this discussion, key benefits of serialization have been underscored, including:
- Improved supply chain efficiency
- Enhanced product safety
- The capability to swiftly manage product recalls
Furthermore, the integration of cutting-edge technologies such as blockchain and IoT devices has revolutionized data management, enabling more precise tracking and transparency. These advancements not only fulfill regulatory requirements but also respond to the growing consumer demand for authenticity and safety in pharmaceutical products.
As the pharmaceutical landscape continues to evolve, embracing serialization is essential for manufacturers aiming to thrive in a competitive environment. By prioritizing innovative tracking solutions and robust data management practices, companies can safeguard their products and foster long-lasting relationships with their customers. The call to action is clear: investing in serialization technologies today is not merely about compliance; it is a strategic move towards building a more transparent, trustworthy, and efficient pharmaceutical industry for the future.
Frequently Asked Questions
What is Western Packaging's role in the pharmaceutical sector?
Western Packaging provides integrated serialization solutions that enhance the packaging and distribution processes for pharmaceutical items, particularly in the nutraceutical sector. They offer expertise in packaging design, filling services, and comprehensive third-party logistics (3PL) services, facilitating seamless product tracking in the supply chain.
How does serialization benefit the pharmaceutical industry?
Serialization assigns a unique identifier to each saleable unit, allowing manufacturers to track products throughout the supply chain. This enhances transparency, accountability, and authenticity while helping to combat counterfeit drugs, which is a significant threat to patient safety.
Why is serialization important for compliance?
Serialization is a regulatory requirement in over 100 nations, compelling pharmaceutical firms to invest in serialization systems to maintain compliance and protect their integrity, especially for smaller distributors who may face challenges in adopting these technologies.
What are the consequences of counterfeit drugs in the market?
Counterfeit drugs pose a substantial threat to patient safety and can lead to significant financial losses for pharmaceutical companies. The World Health Organization estimates that 4 out of 10 medications in developing nations may be contaminated.
How does Western Packaging ensure compliance and operational efficiency?
Western Packaging's proactive approach includes implementing data encoding to reduce counterfeit risks, improve inventory management, and enhance operational efficiency, ensuring that clients remain compliant with evolving regulations.
What is the significance of unique identifiers in serialization?
Unique identifiers enable precise product tracking and monitoring, making it difficult for counterfeit drugs to enter the market and ensuring that only authorized trading partners are involved in the distribution process.
Can you provide an example of successful serialization implementation?
The United States Drug Supply Chain Security Act (DSCSA) has established a framework for identifying and tracing prescription drugs, significantly reducing counterfeit incidents. Additionally, Turkey's Pharmaceutical Track and Trace System has improved oversight and reduced counterfeit occurrences by over 30%.
What should producers consider when implementing data encoding?
Producers should invest in robust tracking systems and collaborate with regulatory organizations to ensure compliance and enhance consumer confidence in their products.




