Overview
Choosing the right nutraceutical contract manufacturing partner hinges on several key factors:
- Regulatory compliance
- Quality assurance practices
- Communication capabilities
- Scalability
- Transparency in sourcing
- Customization expertise
- Track record
- Understanding of manufacturing processes
- Post-production support
By prioritizing these elements, companies can ensure product safety, enhance brand reputation, and foster reliable partnerships. This strategic approach is essential for achieving success in the competitive nutraceutical market. Emphasizing these factors not only solidifies trust but also positions businesses for long-term growth and stability.
Introduction
Selecting the appropriate nutraceutical contract manufacturer is a pivotal decision that can profoundly influence product quality and brand reputation. As the nutraceutical industry undergoes rapid expansion, businesses must adeptly navigate a multifaceted landscape characterized by compliance, quality assurance, and cutting-edge manufacturing capabilities. This article explores ten essential factors that should inform this selection process, offering insights designed to empower companies in establishing successful partnerships.
How can businesses ensure they not only comply with regulatory standards but also bolster their competitive advantage in a saturated market?
Western Packaging: Comprehensive Contract Manufacturing Services for Nutraceuticals
Western Packaging & Distribution stands as a leader in providing a comprehensive suite of nutraceutical contract manufacturing services tailored specifically for the industry. With a wealth of expertise in innovative packaging design and integrated filling services for various formats—including powders, gummies, and soft-gels—Western Packaging excels in delivering robust third-party logistics (3PL). By optimizing warehousing, inventory management, and logistics, this integrated approach not only streamlines the supply chain but also enhances product appeal. As a result, Western Packaging has become the preferred partner for businesses aiming to elevate their packaging and distribution capabilities. Choose Western Packaging & Distribution for reliable solutions that drive success in nutraceutical contract manufacturing.
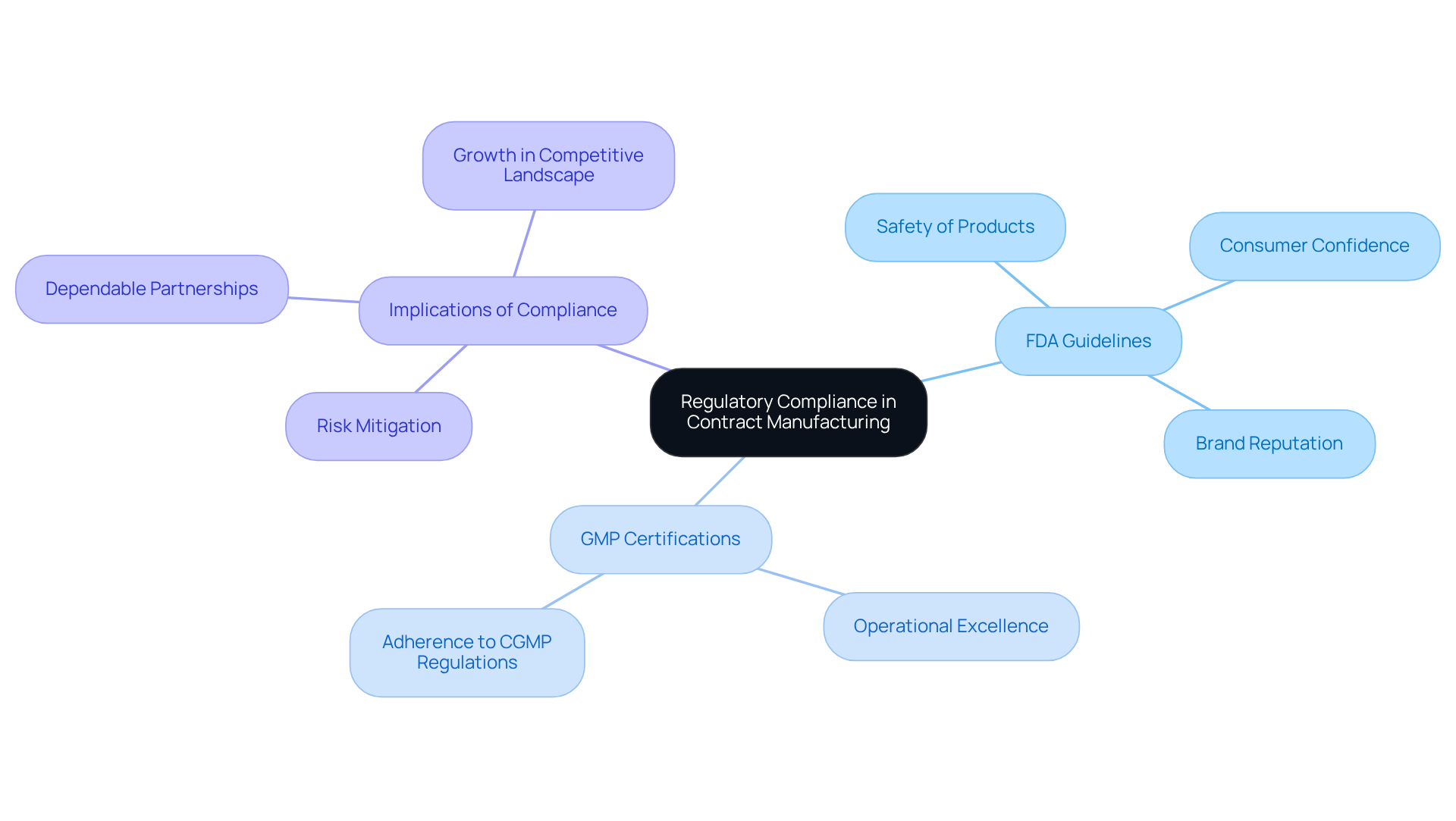
Prioritize Regulatory Compliance in Your Contract Manufacturer Selection
When selecting a contract producer, prioritizing regulatory compliance is paramount. It is essential to ensure that the manufacturer adheres to FDA guidelines and possesses certifications such as Good Manufacturing Practices (GMP). This adherence not only guarantees the safety of products but also enhances consumer confidence and bolsters brand reputation.
The FDA has the authority to alert the public and confiscate medications if a company refuses to withdraw a product, underscoring the critical importance of regulatory compliance. Furthermore, GMP certification reflects a producer's commitment to operational excellence, ensuring that processes align with the stringent Current Good Manufacturing Practices (CGMP) regulations that safeguard the identity, strength, quality, and purity of health products.
By placing these compliance factors at the forefront, businesses can mitigate risks and cultivate a dependable partnership that supports their growth within the competitive landscape of nutraceutical contract manufacturing.
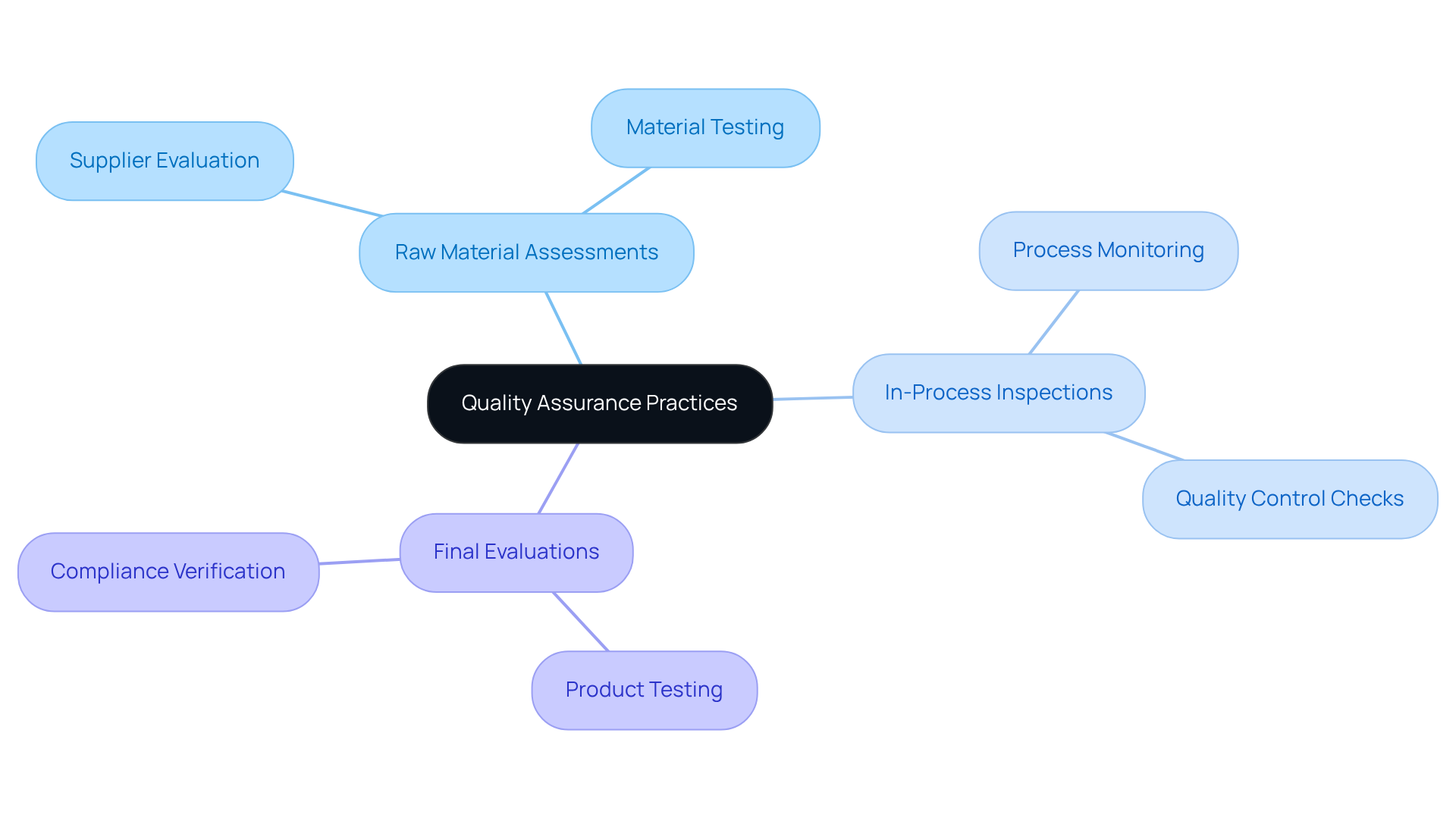
Evaluate Quality Assurance Practices of Potential Manufacturers
Quality assurance must be prioritized when evaluating potential manufacturers. Seek firms that implement rigorous testing procedures, including:
- Raw material assessments
- In-process inspections
- Final evaluations
A robust quality assurance program not only minimizes the risk of defects but also ensures that products meet consumer expectations, thereby enhancing reliability and trust in the manufacturing process.
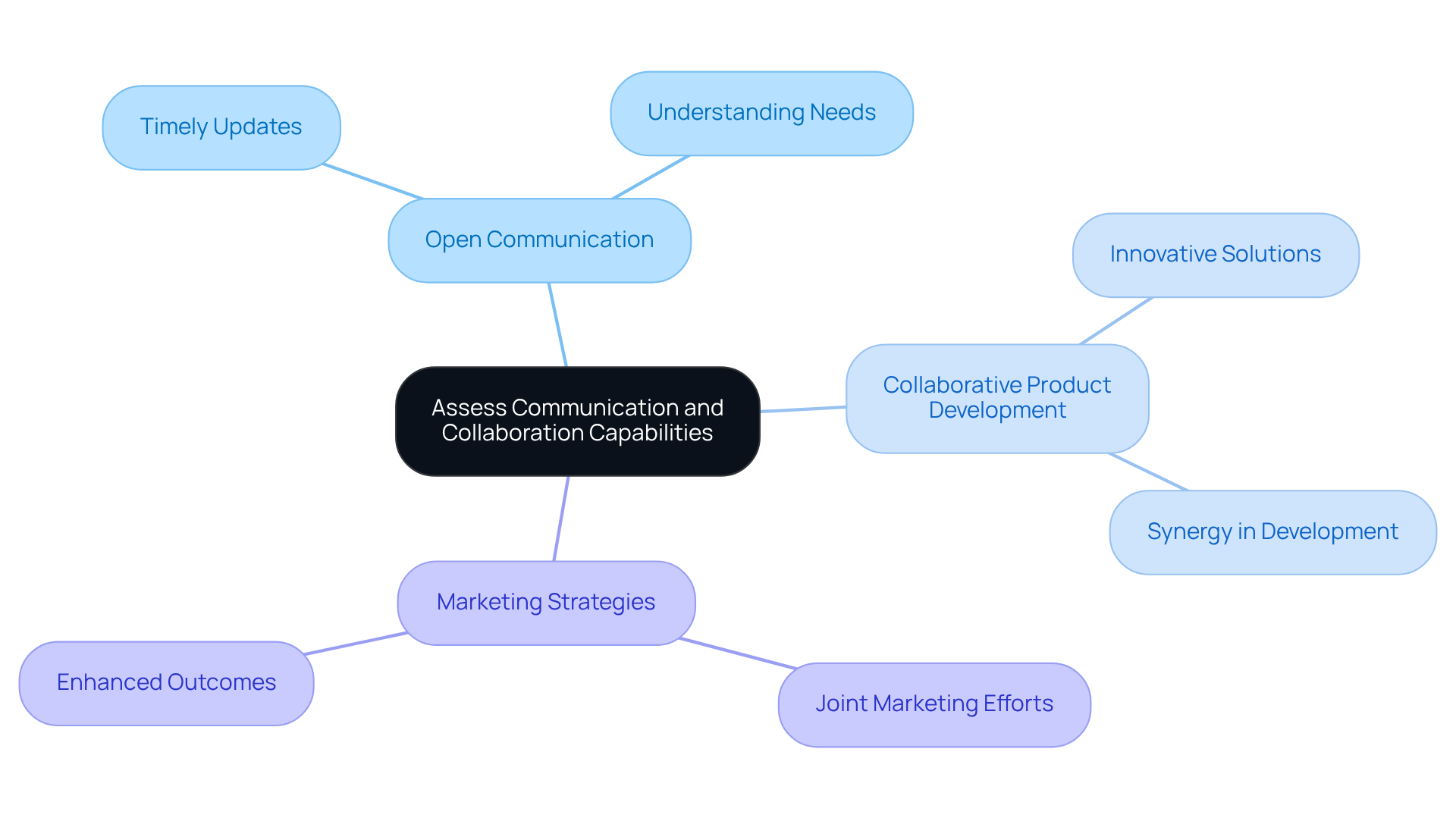
Assess Communication and Collaboration Capabilities of Manufacturers
Evaluate the communication and collaboration abilities of potential suppliers. A producer that fosters open communication can more effectively understand your needs and provide timely updates throughout the production process. Seek partners who are eager to collaborate on product development and marketing strategies, as this synergy can lead to innovative solutions and enhanced outcomes.
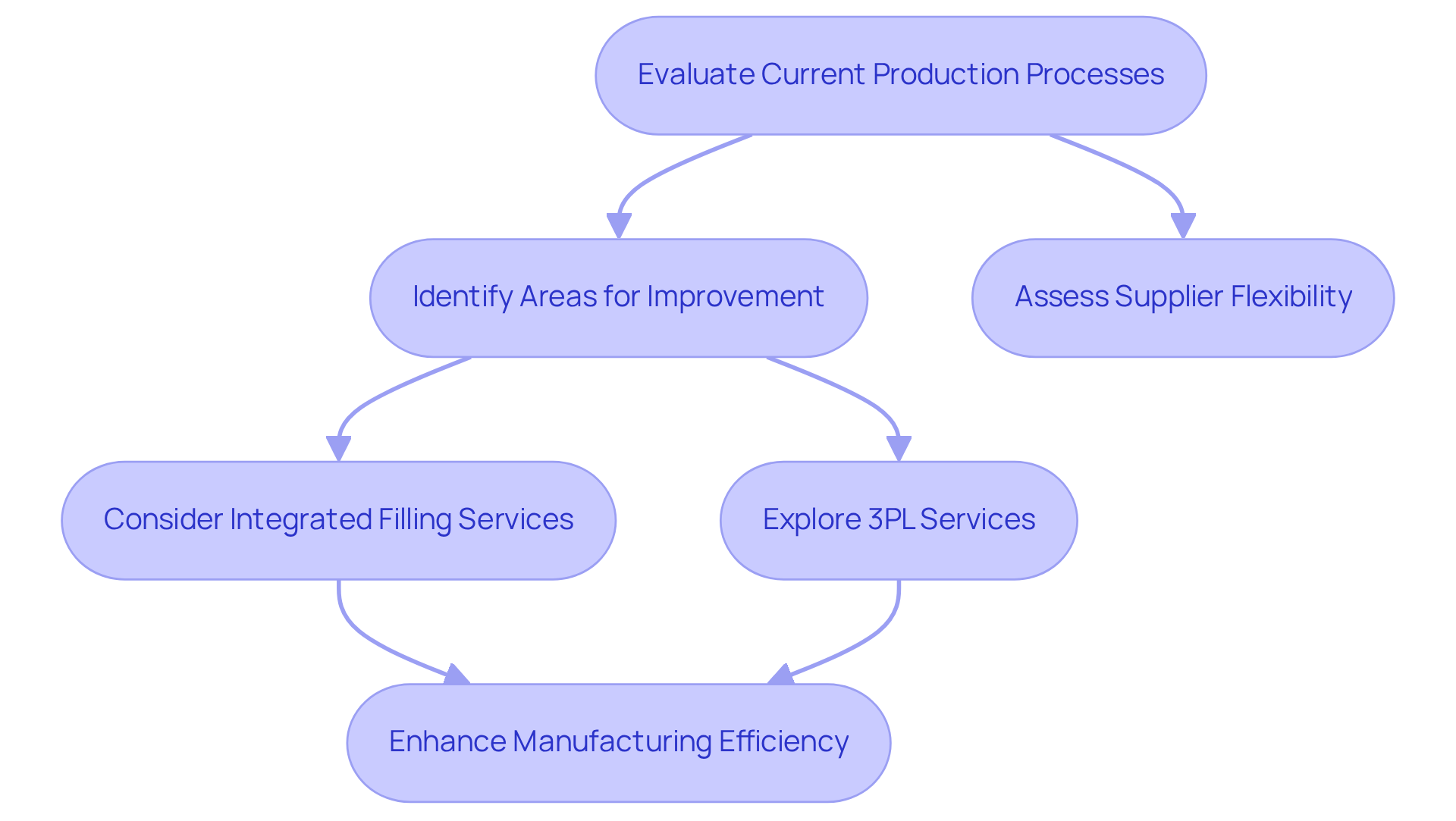
Consider Scalability and Flexibility in Manufacturing Capacity
Consider the scalability and flexibility of a producer's production capacity. As your business expands or encounters seasonal demand increases, it is crucial that your supplier can adjust production levels accordingly.
Western Packaging's integrated filling services—ranging from powders to gummies and soft-gels—seamlessly integrate into your production process, offering flexible packaging solutions tailored to your specific needs.
Furthermore, our comprehensive 3PL services optimize your supply chain management, ensuring that you can meet market demands without delays.
To enhance your scalability, it is advisable to evaluate your current production processes to identify areas where integrated filling and 3PL services can significantly enhance efficiency.
Ensure Transparency in Ingredient Sourcing and Supply Chain
Ensure that your contract producer maintains transparency in ingredient sourcing and supply chain practices. By understanding the origin of ingredients and their processing methods, you can effectively steer clear of potential quality concerns. This practice not only safeguards your product's integrity but also aligns with consumer preferences for ethically sourced items, enhancing your brand's reputation. Emphasizing transparency is not merely a best practice; it is a critical component in today's market that demands accountability and ethical considerations.
Look for Customization and Formulation Expertise in Your Partner
When selecting a nutraceutical contract manufacturing producer, it is essential to prioritize those that offer customization and formulation expertise. The ability to create unique formulations tailored to your target audience can set your nutraceutical contract manufacturing offerings apart in a competitive market. A manufacturer engaged in nutraceutical contract manufacturing with a robust R&D team can drive innovation and address specific consumer needs.
Consider collaborating with a partner like Western Packaging, which specializes in customized flexible packaging solutions, including:
- Large pouches for protein products
- Stick packs for nutraceuticals
Their innovative packaging design not only enhances product appeal but also boosts brand recognition, ensuring your products stand out on the shelf. By partnering with Western Packaging, you can leverage their expertise to elevate your brand's visibility in the health product market.
Investigate Track Record and Client References of Manufacturers
When selecting a nutraceutical contract manufacturing producer, it is crucial to conduct a thorough investigation into their track record and client references. Seek out testimonials and case studies that demonstrate their consistent ability to deliver high-quality products on schedule. A solid reputation within the industry typically signifies a dependable partner capable of meeting your specific requirements.
Testimonials serve as powerful endorsements, showcasing real-world experiences from other clients, which provide valuable insights into the producer's operational capabilities and commitment to excellence. Furthermore, evaluating client references enables you to assess the manufacturer's responsiveness, communication effectiveness, and overall satisfaction among their clientele. This due diligence not only mitigates risks associated with outsourcing but also ensures that you choose a partner who aligns with your standards and business objectives.
As Mathias Buchmann observes, "At SMT, we’ve established stringent control measures and cutting-edge testing procedures to guarantee each item meets or surpasses industry standards."
Additionally, understanding potential communication barriers stemming from language and cultural differences is vital in evaluating producers, as these factors can significantly impact collaboration and project success. Risks related to standards are inherent in nutraceutical contract manufacturing, making it imperative to thoroughly assess a manufacturer's reputation to avoid issues that could compromise your product's quality and marketability.
Understand the Manufacturing Process and Capabilities Offered
Gaining a comprehensive understanding of the manufacturing processes and capabilities of potential nutraceutical contract manufacturing partners is crucial. This involves familiarizing yourself with the types of equipment utilized, the production techniques implemented, and any specialized processes pertinent to your products. For example, advanced machinery such as encapsulators, mixers, and filling lines can significantly influence both the quality and efficiency of production. By ensuring that the manufacturing capabilities align with your specifications, you can avert potential misalignments that could jeopardize the integrity of the product.
Moreover, recognizing the importance of efficient operational strategies is essential; successful manufacturing hinges on the seamless integration of personnel, products, and profit. As Patrick Lencioni asserts, an organization possesses integrity when its management, operations, strategy, and culture harmonize effectively. This holistic approach not only enhances operational efficiency in nutraceutical contract manufacturing but also fosters innovation, ultimately leading to superior offerings in the competitive health supplement market.
To further ensure alignment, consider conducting site visits to evaluate equipment and processes firsthand.
Evaluate Post-Production Support and Commitment to Excellence
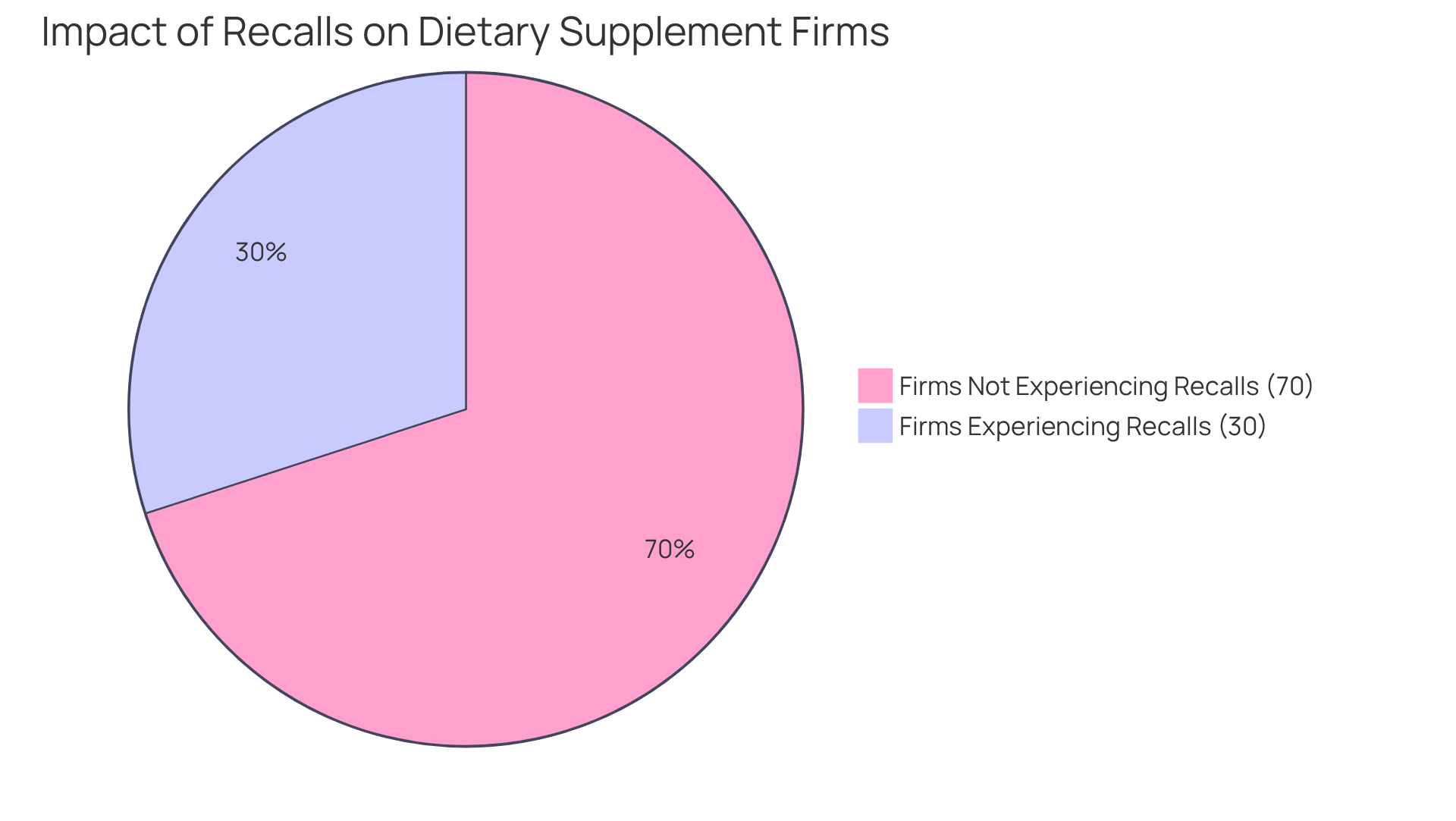
Evaluating the post-production assistance provided by prospective manufacturers is essential for ensuring the success of health supplements. A commitment to excellence must extend beyond the production phase, encompassing critical areas such as recalls, assurance of standards, and ongoing regulatory compliance. In the dietary supplement sector, where recalls can significantly damage a brand's image, robust post-manufacturing support is paramount. Data indicate that nearly 30% of dietary supplement firms experience product recalls due to standards concerns, underscoring the necessity for producers to establish effective protocols. This statistic is derived from industry reports highlighting the risks associated with insufficient post-production support.
Strong post-production support not only mitigates the risks associated with recalls but also enhances brand credibility. Companies that prioritize management systems and uphold stringent testing protocols are better positioned to address potential issues swiftly, thereby maintaining consumer confidence. A notable example is XYZ Corp, a leading health supplement manufacturer that implemented a comprehensive post-production quality control system, resulting in a 50% reduction in recalls over two years. Their strategy included enhanced testing procedures and regular audits to ensure compliance with FDA guidelines and CGMP standards.
Ultimately, a dedication to post-production excellence in nutraceutical contract manufacturing can significantly impact your brand's reputation and long-term success in the competitive nutraceutical market.
Conclusion
Choosing the right nutraceutical contract manufacturer is a critical decision that can significantly influence the success of a brand. By focusing on key factors such as regulatory compliance, quality assurance, and effective communication, businesses can forge partnerships that not only meet but exceed industry standards. The insights provided in this article serve as a roadmap for navigating the complexities of nutraceutical manufacturing, ensuring that companies select a manufacturer capable of supporting their growth and maintaining product integrity.
Essential considerations have been highlighted throughout this discussion, including the importance of:
- Scalability
- Transparency in ingredient sourcing
- The need for robust post-production support
By prioritizing these elements, brands can minimize risks, enhance consumer trust, and ultimately achieve a competitive edge in the nutraceutical market. Furthermore, the emphasis on customization and formulation expertise underscores the necessity of collaborating with a manufacturer who can adapt to specific market demands and consumer preferences.
In conclusion, the journey of selecting a nutraceutical contract manufacturer should be approached with diligence and strategic foresight. By leveraging the insights shared in this article, businesses can make informed decisions that align with their goals and values. A commitment to excellence in every aspect of the manufacturing process will not only bolster brand reputation but also pave the way for sustained success in the dynamic landscape of health products.
Frequently Asked Questions
What services does Western Packaging provide for nutraceuticals?
Western Packaging offers a comprehensive suite of contract manufacturing services specifically tailored for the nutraceutical industry, including innovative packaging design, integrated filling services for formats like powders, gummies, and soft-gels, as well as robust third-party logistics (3PL).
How does Western Packaging enhance the supply chain for businesses?
Western Packaging optimizes warehousing, inventory management, and logistics, which streamlines the supply chain and enhances product appeal, making it a preferred partner for businesses in the nutraceutical sector.
Why is regulatory compliance important when selecting a contract manufacturer?
Regulatory compliance is crucial because it ensures that the manufacturer adheres to FDA guidelines and possesses certifications like Good Manufacturing Practices (GMP), which guarantees product safety, enhances consumer confidence, and bolsters brand reputation.
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are regulations that ensure manufacturers align their processes with stringent standards to safeguard the identity, strength, quality, and purity of health products.
What quality assurance practices should be evaluated in potential manufacturers?
When evaluating potential manufacturers, it is important to seek firms that implement rigorous testing procedures, including raw material assessments, in-process inspections, and final evaluations to minimize defects and ensure product reliability.
How does a robust quality assurance program benefit the manufacturing process?
A robust quality assurance program minimizes the risk of defects and ensures that products meet consumer expectations, thereby enhancing reliability and trust in the manufacturing process.




