Overview
Ensuring packaging safety in nutraceuticals is paramount, and best practices in this field include:
- A thorough understanding of regulatory compliance
- The selection of safe materials
- The implementation of robust quality control measures
- The optimization of supply chain logistics
Adherence to FDA regulations is crucial, as is the careful selection of appropriate packaging materials. Regular audits and effective logistics strategies are essential to maintain product integrity and foster consumer trust in the nutraceutical industry. By prioritizing these practices, companies can not only meet regulatory standards but also enhance their reputation and reliability in the marketplace.
Introduction
Ensuring the safety of nutraceutical packaging transcends mere regulatory obligation; it stands as a critical pillar in upholding product integrity and fostering consumer trust. As the demand for nutraceuticals escalates, the necessity for adherence to best practices in packaging safety becomes paramount. This article explores essential strategies that manufacturers can employ to navigate the intricacies of regulatory compliance, select safe materials, enforce rigorous quality control, and optimize supply chain logistics.
With the landscape of regulations and consumer expectations continuously evolving, the pressing question remains: how can companies stay ahead and guarantee that their packaging not only meets safety standards but also aligns with market demands?
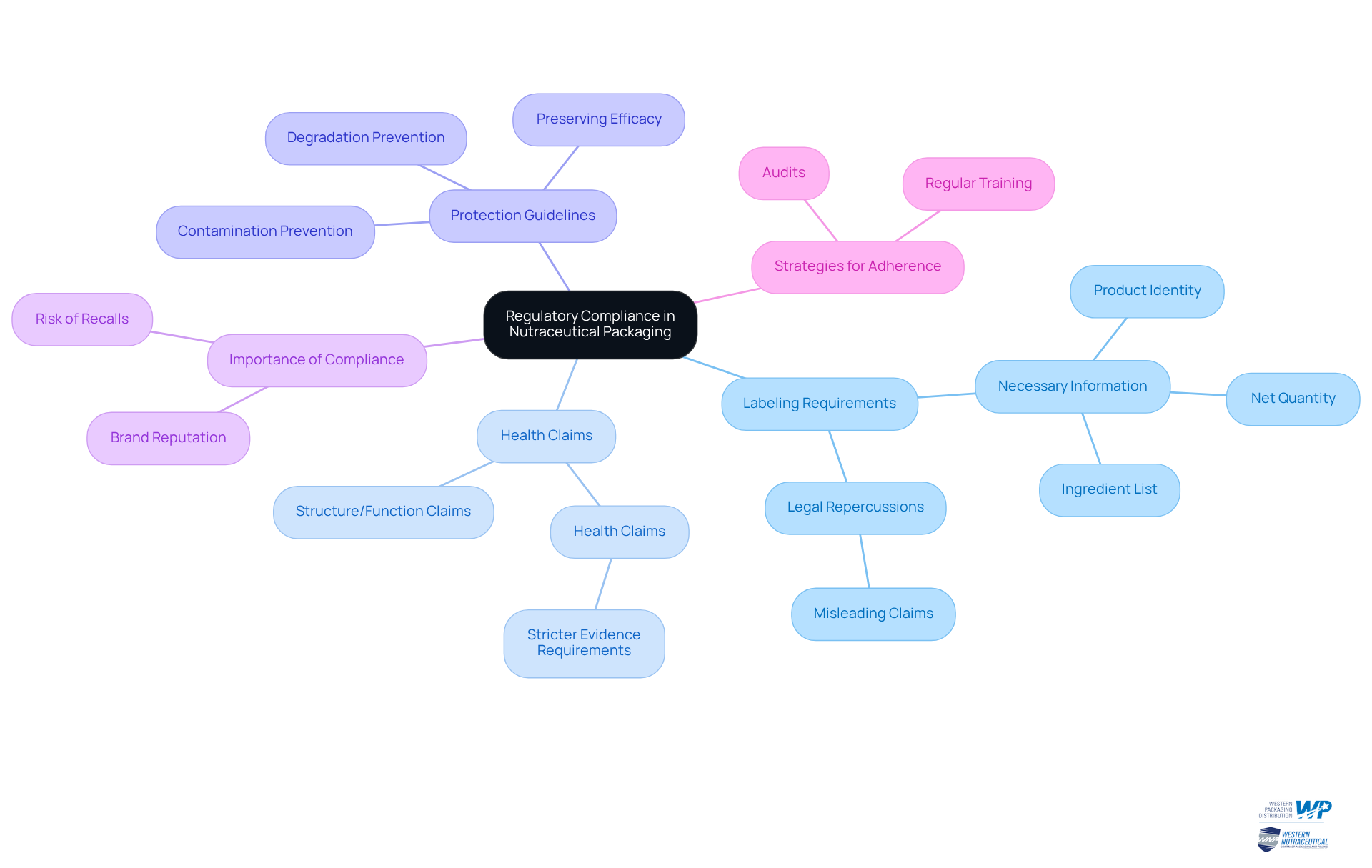
Understand Regulatory Compliance in Nutraceutical Packaging
Nutraceutical packaging safety must adhere to various regulations, including the FDA's Current Good Manufacturing Practices (cGMPs) and labeling requirements. This compliance is not merely a recommendation; it is essential for maintaining product integrity and protecting brand reputation. Key areas to focus on include:
- Labeling Requirements: Ensure that all labels include necessary information such as product identity, net quantity, and ingredient list. Misleading claims can lead to legal repercussions.
- Health Claims: Understand the difference between structure/function claims and health claims, as the latter requires more stringent evidence.
- Protection Guidelines: Packaging safety is crucial as it must safeguard the item from contamination and degradation, which is essential for preserving efficacy.
Staying informed about regulatory changes is crucial. Non-compliance can lead to recalls and significant harm to brand reputation. Regular training and audits are vital strategies to ensure adherence to these regulations, reinforcing your commitment to quality and reliability in nutraceutical packaging.
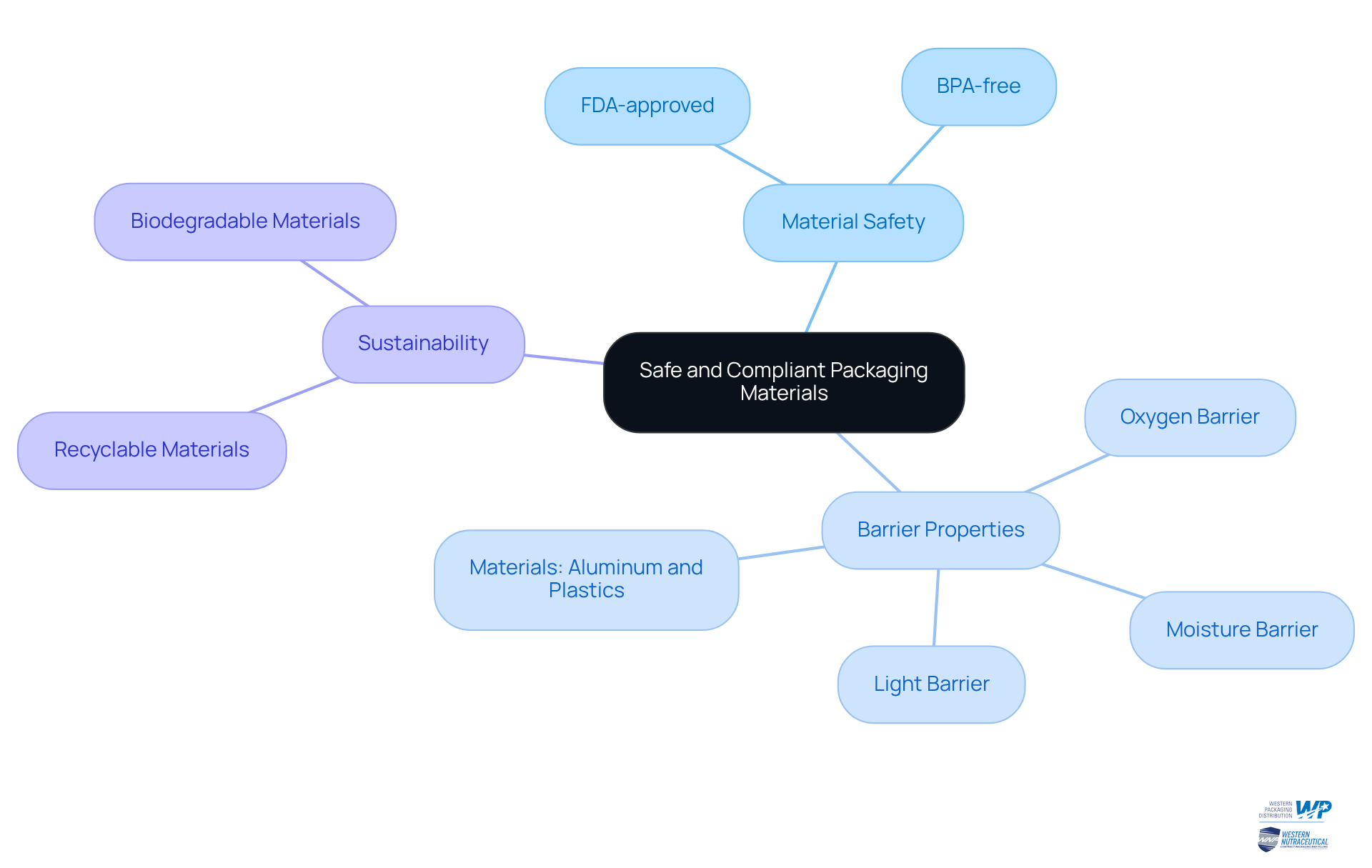
Choose Safe and Compliant Packaging Materials
When selecting packaging materials for nutraceuticals, it is essential to consider the following factors:
- Material Safety: It is crucial to ensure that materials are FDA-approved and BPA-free to prevent harmful chemical leaching into products.
- Barrier Properties: Opt for substances that provide adequate barriers against moisture, oxygen, and light, which can compromise product quality. For example, aluminum and certain plastics are excellent choices for preserving the stability of items.
- Sustainability: Given the increasing consumer demand for environmentally friendly options, it is advisable to utilize recyclable or biodegradable materials that align with sustainability objectives.
Conducting thorough evaluations of container materials can help identify potential interactions with the product, ensuring safety and compliance.
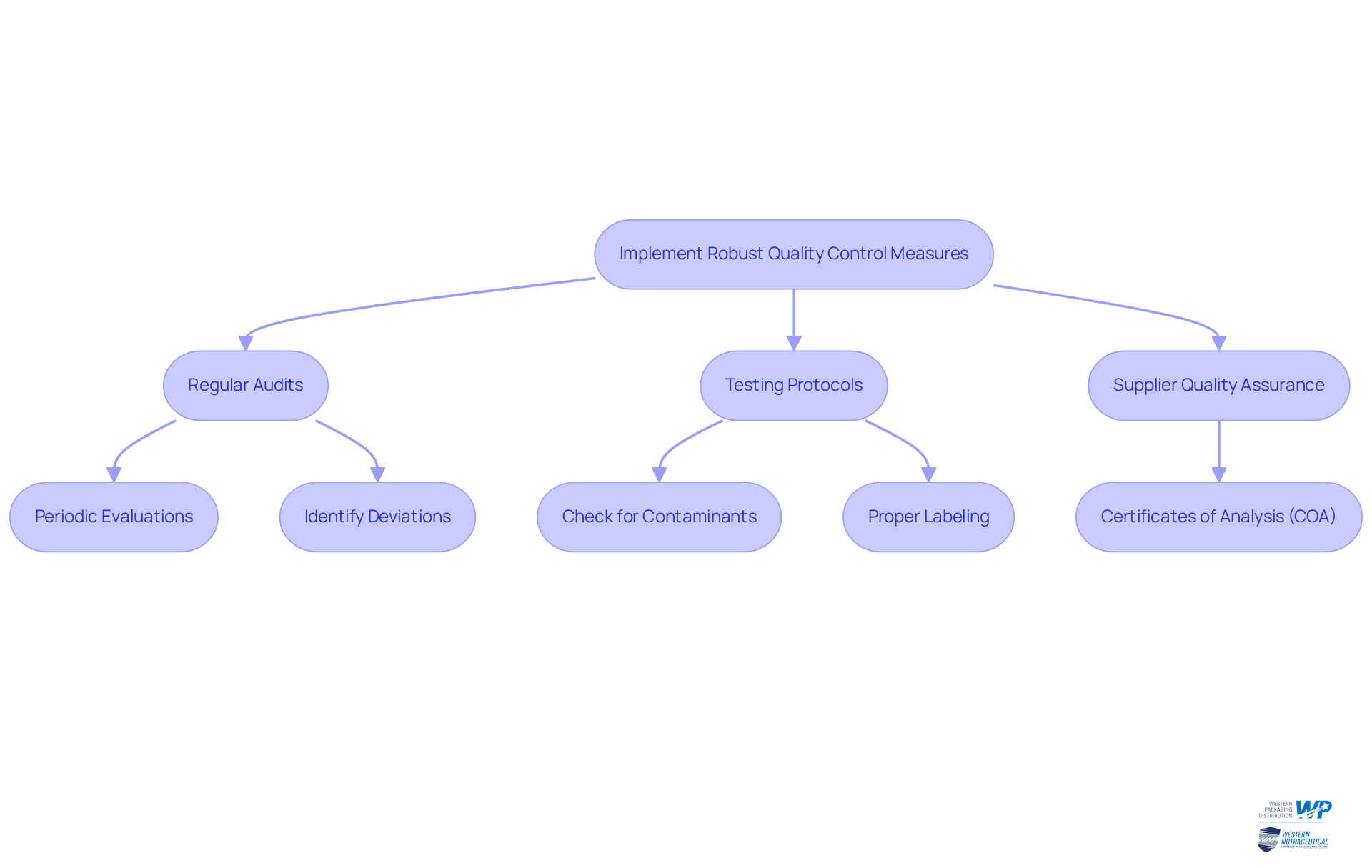
Implement Robust Quality Control Measures
To guarantee the packaging safety and quality of nutraceuticals, it is imperative to implement rigorous quality control measures.
-
Regular Audits: Conduct periodic evaluations of packaging processes to identify and rectify any deviations from established standards. These audits ensure that every aspect of the packaging meets the highest benchmarks of quality and packaging safety.
-
Testing Protocols: Develop comprehensive testing protocols for both packaging substances and completed items. This ensures adherence to packaging safety and performance standards, including checks for contaminants and proper labeling, which are vital for consumer protection.
-
Supplier Quality Assurance: Collaborate closely with suppliers to ensure compliance with established standards. It is essential that suppliers provide Certificates of Analysis (COA) for raw materials, thereby reinforcing the integrity of the entire supply chain.
By fostering a culture of excellence within the organization, manufacturers can significantly enhance reliability and strengthen consumer trust.
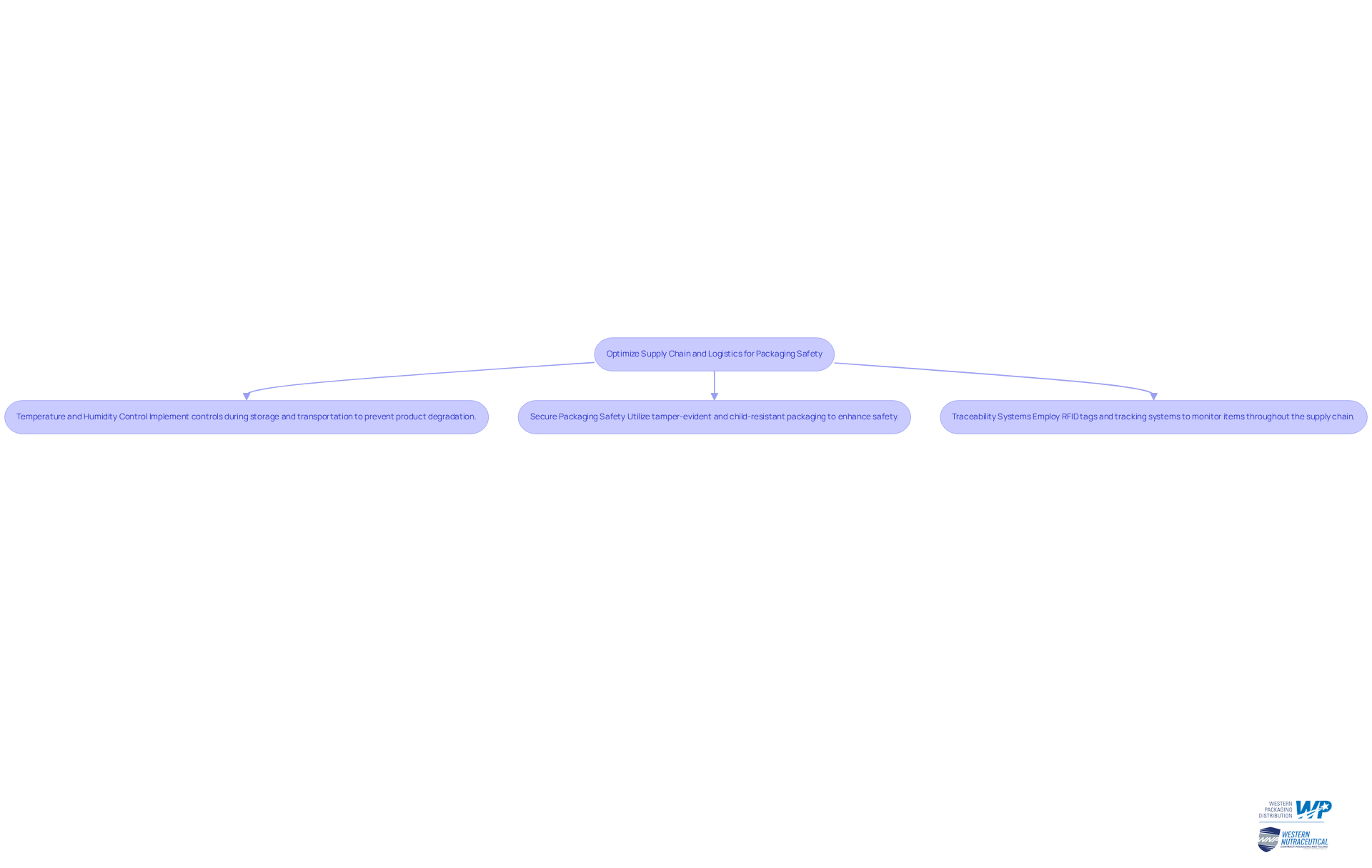
Optimize Supply Chain and Logistics for Packaging Safety
To optimize supply chain and logistics for packaging safety, consider the following strategies:
- Temperature and Humidity Control: Implement temperature and humidity controls during storage and transportation to prevent product degradation. This is particularly crucial for sensitive nutraceuticals, ensuring their efficacy and safety.
- Secure Packaging Safety: Utilize tamper-evident and child-resistant packaging to enhance safety during distribution. Such measures not only safeguard the item but also foster consumer confidence, reinforcing brand integrity.
- Traceability Systems: Employ RFID tags and tracking systems to monitor items throughout the supply chain. This ensures that any issues can be swiftly identified and addressed, maintaining operational efficiency.
By focusing on these logistics strategies, manufacturers can significantly reduce the risk of product damage and ensure packaging safety compliance with safety regulations. Take action now to improve your packaging safety and reinforce the reliability of your supply chain.
Conclusion
Ensuring packaging safety in nutraceuticals is paramount for maintaining product quality and consumer trust. Adhering to regulatory compliance, selecting safe materials, implementing stringent quality control measures, and optimizing supply chain logistics are essential practices that contribute to the overall integrity of nutraceutical products. By prioritizing these aspects, companies not only safeguard their products but also reinforce their commitment to consumer safety and satisfaction.
Key arguments outlined in this article highlight the importance of understanding regulatory frameworks, such as the FDA's guidelines, and the necessity of using materials that are both safe and environmentally sustainable. Additionally, robust quality control measures, including regular audits and supplier assurance, play a critical role in maintaining high standards throughout the packaging process. Optimizing logistics further enhances safety by addressing factors like temperature control and traceability, ensuring that products remain effective and safe from production to consumption.
In a landscape where consumer awareness is growing, the significance of packaging safety cannot be overstated. Companies are encouraged to take proactive steps in implementing these best practices, not only to comply with regulations but to build a reputation for reliability and excellence in the nutraceutical market. Embracing these strategies will not only protect brands from potential risks but also position them as leaders in quality and safety in an increasingly competitive industry.
Frequently Asked Questions
What are the key regulations that nutraceutical packaging must adhere to?
Nutraceutical packaging must comply with various regulations, including the FDA's Current Good Manufacturing Practices (cGMPs) and labeling requirements.
Why is regulatory compliance important in nutraceutical packaging?
Compliance is essential for maintaining product integrity and protecting brand reputation. Non-compliance can lead to recalls and significant harm to the brand.
What are the necessary components that must be included on nutraceutical labels?
Labels must include necessary information such as product identity, net quantity, and ingredient list. Misleading claims can result in legal repercussions.
What is the difference between structure/function claims and health claims in nutraceutical packaging?
Structure/function claims describe the role of a nutrient or dietary ingredient in supporting normal body function, while health claims require more stringent evidence to substantiate their accuracy.
Why is packaging safety crucial for nutraceutical products?
Packaging safety is crucial because it must protect the product from contamination and degradation, which is essential for preserving the product's efficacy.
How can companies ensure they stay informed about regulatory changes?
Companies should engage in regular training and audits to ensure adherence to regulations and stay informed about any changes in compliance requirements.




