Overview
This article highlights four essential bio-pharma shipping solutions that guarantee compliance and efficiency in the transportation of pharmaceutical products:
- Understanding regulatory requirements is paramount.
- Selecting appropriate packaging is crucial.
- Implementing efficient logistics strategies is necessary.
- Maintaining temperature control during transit is vital.
Each of these elements plays a critical role in safeguarding product integrity and meeting industry standards. By prioritizing these strategies, stakeholders can ensure their operations align with best practices in the bio-pharma sector.
Introduction
Navigating the complex landscape of bio-pharma shipping demands a keen understanding of regulatory compliance and efficient logistics strategies. With the surging demand for biologics, companies encounter the dual challenge of adhering to stringent regulations while ensuring the timely delivery of temperature-sensitive products. This article explores four essential shipping solutions that not only enhance compliance but also streamline operations. It prompts a crucial question: how can organizations effectively balance the imperative of regulatory adherence with the need for operational efficiency in an ever-evolving industry?
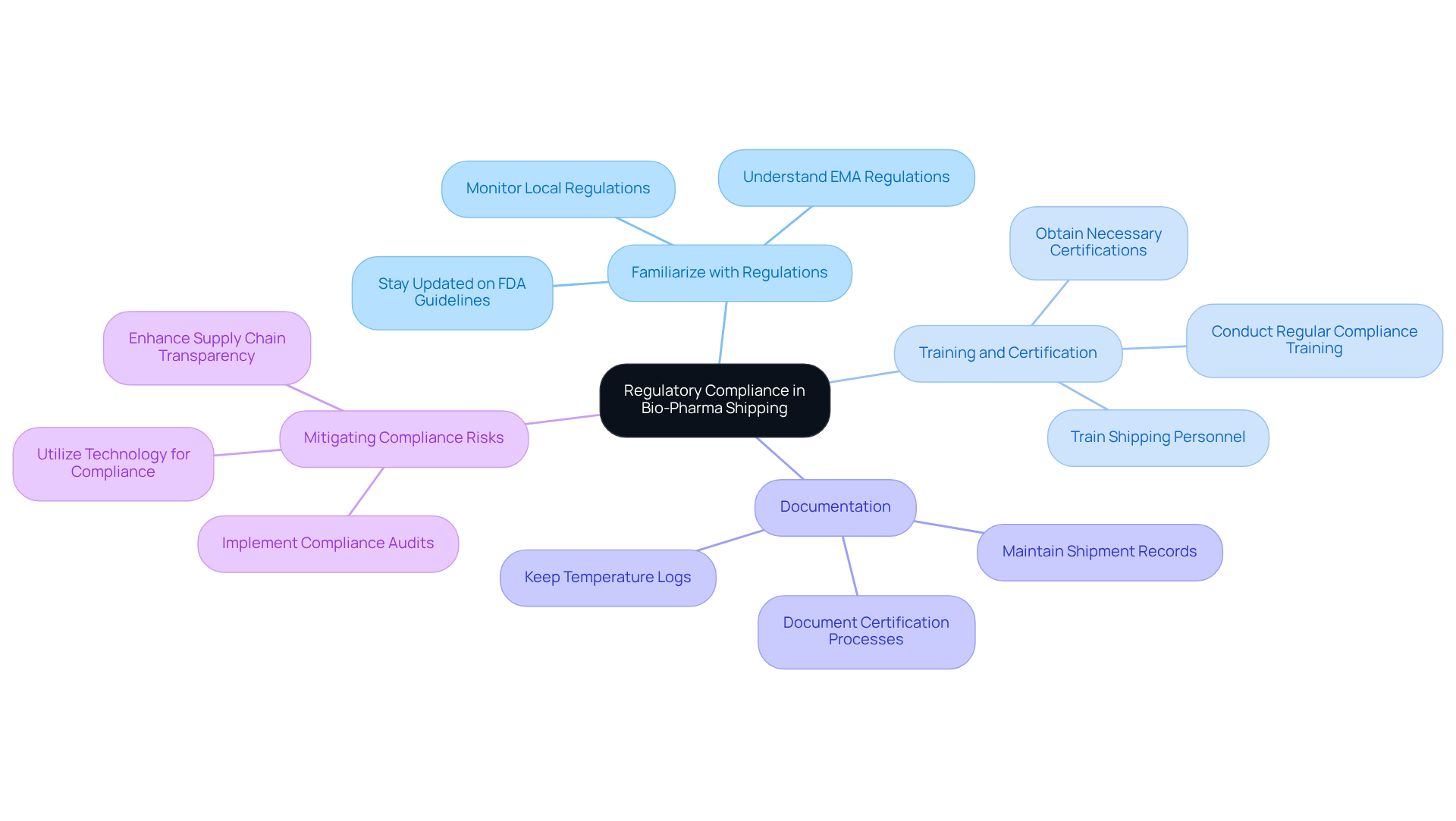
Understand Regulatory Compliance in Bio-Pharma Shipping
Regulatory compliance in pharmaceutical transport is critical, necessitating a thorough understanding and adherence to various local and international regulations, including FDA guidelines in the U.S. and EMA regulations in Europe. Businesses must ensure that their delivery methods align with these standards to avoid fines and guarantee the safety and effectiveness of their products. Key steps to achieving compliance include:
- Familiarize with Regulations: Staying updated on the latest regulations affecting bio-pharma shipping, particularly those related to labeling, documentation, and product handling, is essential.
- Training and Certification: It is imperative that all personnel involved in shipping are trained and certified in regulatory practices to uphold compliance.
- Documentation: Maintaining precise records of all shipments, including temperature logs and certification documents, is crucial to demonstrate adherence to regulations.
By implementing these practices, companies can effectively mitigate risks associated with non-compliance, thereby enhancing their reputation in the market.
Choose Suitable Packaging Solutions for Bio-Pharma Products
Choosing suitable packaging options for bio-pharma shipping solutions is essential for ensuring adherence and preserving item integrity. Key considerations include:
- Material Selection: Selecting materials that meet regulatory standards and are specifically suited for temperature-sensitive biologics is crucial. Advanced polymers, for instance, provide effective barrier properties while ensuring compliance with safety regulations.
- Tamper-Evident Features: Incorporating tamper-evident seals is vital for enhancing security and fostering consumer trust. These features safeguard the item and adhere to strict regulations requiring clear identification of tampering.
- Custom Design: Tailoring packaging designs to meet the unique requirements of each item is imperative. This approach protects pharmaceutical items from environmental influences during transportation. Insulated containers, for example, are specifically created for temperature-sensitive items, significantly minimizing the risk of spoilage and ensuring compliance with cold chain regulations.
The significance of tamper-evident packaging in the pharmaceutical industry cannot be overstated; it plays a vital role in safeguarding both the product and the consumer. As the demand for biologics continues to rise, implementing bio-pharma shipping solutions will be essential for maintaining compliance and efficiency in the supply chain.
Implement Efficient Logistics Strategies for Timely Delivery
Implementing efficient logistics strategies is essential for optimizing bio-pharma shipping solutions in the delivery processes. The key components include:
- Route Optimization: Employ advanced software tools to analyze and select the most efficient shipping routes. These tools consider factors such as traffic patterns and weather conditions, significantly enhancing delivery efficiency. Companies utilizing route optimization software have reported reductions in delivery times by up to 20%, leading to improved operational performance.
- Real-Time Tracking: Investing in tracking technologies that provide real-time updates on shipment status is essential. This capability enables logistics managers to proactively tackle potential delays, ensuring adherence to stringent regulatory requirements. Organizations that have integrated real-time tracking into their logistics operations have experienced a notable decrease in delivery delays, boosting customer satisfaction and adherence to timelines.
- Collaboration with 3PL Providers: Partnering with third-party logistics providers specializing in the pharmaceutical sector can leverage their expertise and resources, resulting in enhanced operational efficiency. These providers often have established networks and systems that streamline the logistics process, further optimizing delivery times and reducing costs.
By focusing on these strategies, companies can significantly improve their bio-pharma shipping solutions, ensuring timely delivery and compliance with industry standards.
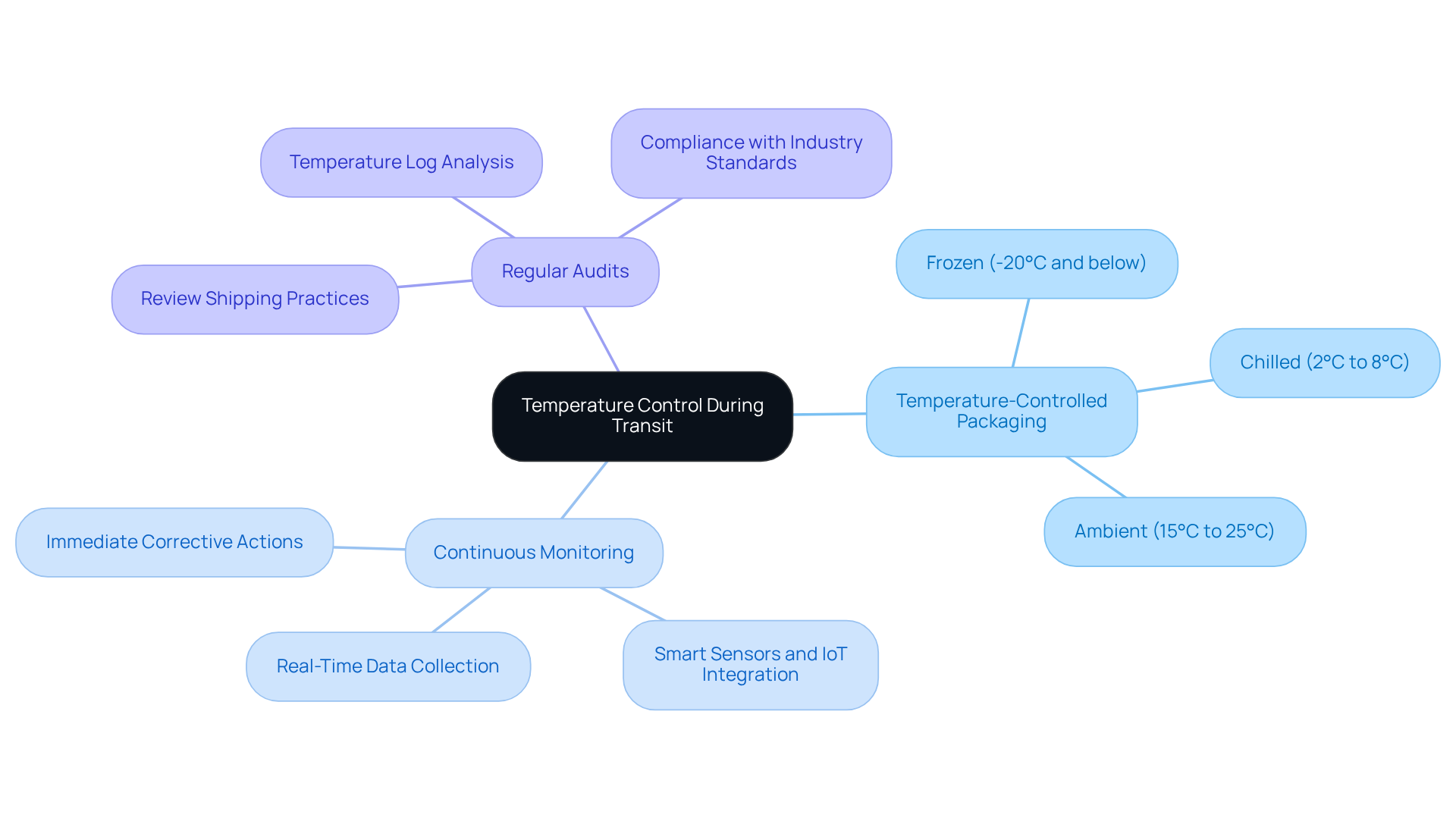
Ensure Temperature Control and Monitoring During Transit
To uphold the integrity of temperature-sensitive items during transit, it is essential to implement effective temperature control and monitoring practices. Here are key strategies to consider:
-
Utilize Temperature-Controlled Packaging: Invest in specialized packaging solutions designed to sustain the required temperature ranges for sensitive products, such as frozen (-20°C and below), chilled (2°C to 8°C), and ambient (15°C to 25°C). This ensures that products remain within safe temperature limits throughout their journey.
-
Implement Continuous Monitoring: Deploy advanced temperature monitoring devices that provide real-time data during transit. These devices alert stakeholders to any temperature deviations, allowing for immediate corrective actions. The integration of smart sensors and IoT technologies enhances the reliability of these monitoring systems, significantly reducing the risk of spoilage.
-
Conduct Regular Audits: Regularly review shipping practices and temperature logs to ensure adherence to industry standards and identify areas for improvement. This proactive approach not only enhances operational efficiency but also aligns with regulatory requirements, which are increasingly stringent regarding bio-pharma shipping solutions.
For instance, companies employing sophisticated temperature monitoring systems have reported a notable decrease in product spoilage rates, translating into substantial cost savings and improved compliance with regulatory mandates. As Ismail Sutaria, Lead Consultant in Packaging, stated, "The Cold Chain Packaging Market is vital for ensuring temperature-sensitive goods, especially in pharmaceuticals and food, maintain their integrity. As demand for secure, effective transport increases, advancements in materials and technologies are essential for industry development." With the global temperature-controlled packaging market projected to reach USD 23.6 billion by 2035 and a CAGR of 5.5% from 2025 to 2035, the emphasis on continuous temperature monitoring will only grow, underscoring its importance in bio-pharma shipping solutions.
Conclusion
Navigating the intricate world of bio-pharma shipping demands a strategic approach that prioritizes regulatory compliance alongside operational efficiency. This article outlines four critical solutions organizations can implement to confront these challenges directly, ensuring adherence to stringent regulations while simultaneously enhancing logistics processes.
Key insights include:
- The necessity of comprehensively understanding and adhering to regulatory standards
- Selecting appropriate packaging solutions
- Optimizing logistics strategies
- Maintaining strict temperature control throughout transit
By familiarizing themselves with regulations, investing in suitable packaging, leveraging advanced logistics technologies, and implementing robust temperature monitoring systems, companies can significantly mitigate risks and elevate their overall shipping efficiency.
As the bio-pharma industry continues to evolve, the importance of compliance and efficiency will only intensify. Organizations are urged to adopt these best practices and remain vigilant about emerging trends in bio-pharma shipping logistics. By doing so, they can ensure the safe and effective delivery of their products, ultimately fostering trust and reliability in a market that demands excellence.
Frequently Asked Questions
Why is regulatory compliance important in bio-pharma shipping?
Regulatory compliance is critical in pharmaceutical transport to ensure adherence to local and international regulations, including FDA guidelines in the U.S. and EMA regulations in Europe. Compliance helps avoid fines and guarantees the safety and effectiveness of pharmaceutical products.
What are some key steps to achieve regulatory compliance in bio-pharma shipping?
Key steps include familiarizing oneself with regulations, ensuring training and certification for personnel involved in shipping, and maintaining precise documentation of all shipments.
How can businesses stay updated on regulations affecting bio-pharma shipping?
Businesses can stay updated by regularly reviewing the latest regulations related to labeling, documentation, and product handling that affect bio-pharma shipping.
Why is training and certification important for personnel involved in shipping?
Training and certification are imperative for ensuring that all personnel involved in shipping are knowledgeable about regulatory practices, which helps uphold compliance.
What type of documentation is necessary for regulatory compliance in bio-pharma shipping?
Necessary documentation includes precise records of all shipments, temperature logs, and certification documents to demonstrate adherence to regulations.
How can companies mitigate risks associated with non-compliance?
By implementing practices such as staying informed about regulations, training personnel, and maintaining accurate documentation, companies can effectively mitigate risks associated with non-compliance and enhance their market reputation.




