Overview
This article delineates four essential practices for effective tablet packaging:
- Regulatory compliance
- Quality control measures
- Innovative packaging solutions
- Streamlined supply chain processes
Each practice is underpinned by comprehensive explanations highlighting their significance. For instance, adherence to FDA guidelines is paramount for ensuring safety and proper labeling. Rigorous quality checks are vital to uphold industry standards, while the use of sustainable materials addresses growing consumer demand. Furthermore, optimizing supply chain efficiency is crucial for enhancing distribution reliability. By implementing these practices, industry professionals can ensure their packaging solutions meet the highest standards of quality and compliance.
Introduction
Navigating the intricate landscape of tablet packaging requires manufacturers to expertly balance regulatory compliance, quality assurance, and innovative design. The stakes are high; effective packaging not only ensures safety and efficacy but also enhances marketability in a fiercely competitive environment. As consumers increasingly demand sustainable and user-friendly options, manufacturers must adapt their practices to meet these evolving expectations while maintaining compliance and efficiency. By exploring key strategies in packaging, brands can unveil significant opportunities to elevate their presence and foster customer trust.
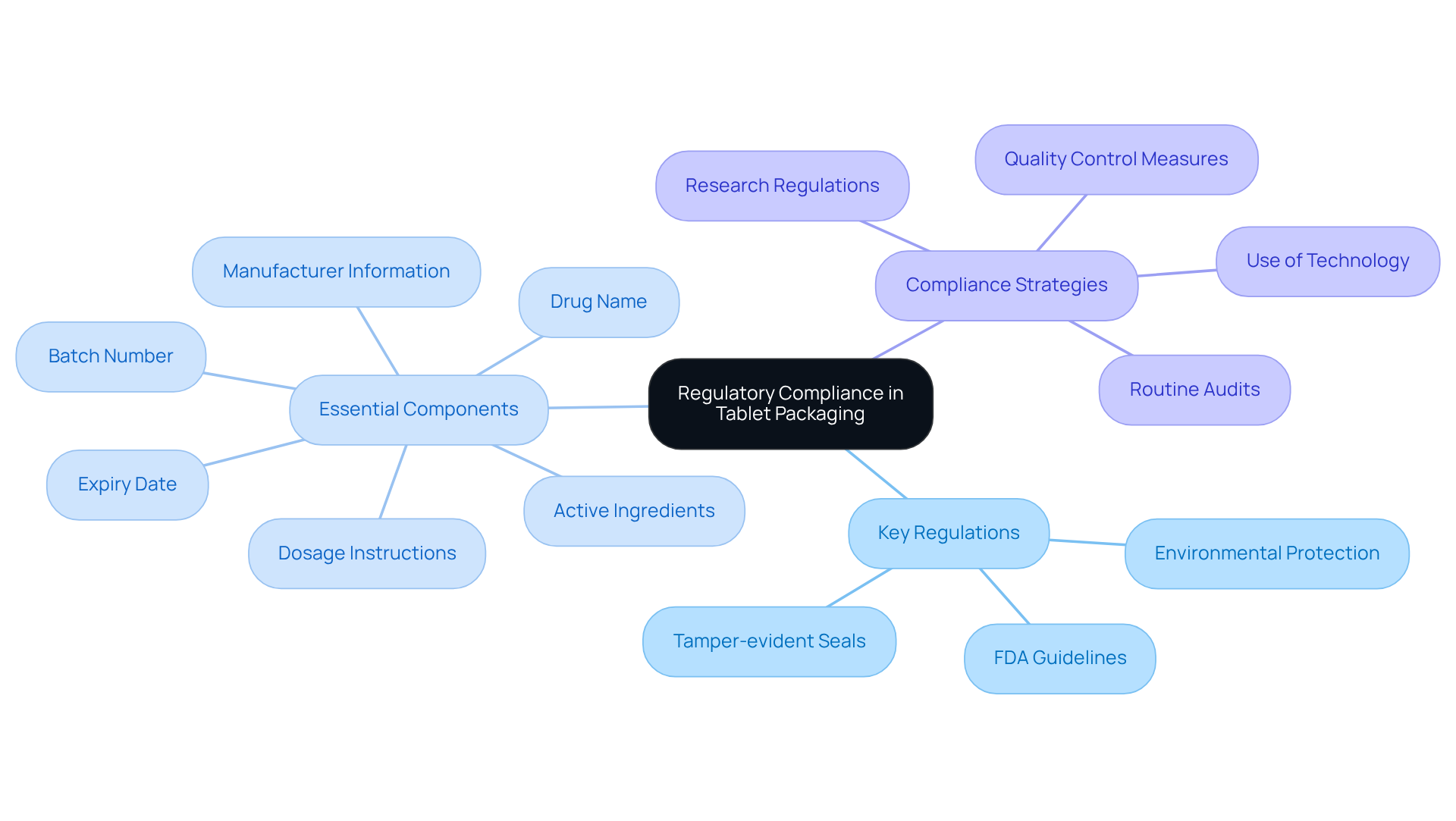
Understand Regulatory Compliance in Tablet Packaging
To effectively manage packaging for tablets, manufacturers must navigate a complex landscape of regulatory requirements. Key regulations encompass the FDA guidelines for labeling and containers, mandating that all materials must be safe, effective, and not misleading. For instance, using tamper-evident seals is a common requirement for over-the-counter medications. Furthermore, the container must shield the product from environmental elements like moisture and light, which can diminish the quality of the tablets. Moreover, it must include essential details such as:
- Drug name
- Dosage instructions
- Active ingredients
- Batch number
- Expiry date
- Manufacturer information
These are critical components for compliance and patient safety.
Manufacturers should conduct thorough research on local and international regulations to ensure compliance, as non-compliance can lead to costly recalls and damage to brand reputation. Quality control measures are vital in preventing mislabeling and ensuring adherence to regulatory standards. Routine audits and discussions with regulatory specialists can assist in maintaining compliance throughout the packing process. Moreover, leveraging technology can enhance container functionality and safety, providing a more comprehensive approach to meeting regulatory requirements. By prioritizing compliance in their packaging for tablets, manufacturers can enhance their market presence and ensure effective product delivery.
Implement Quality Control Measures for Packaging
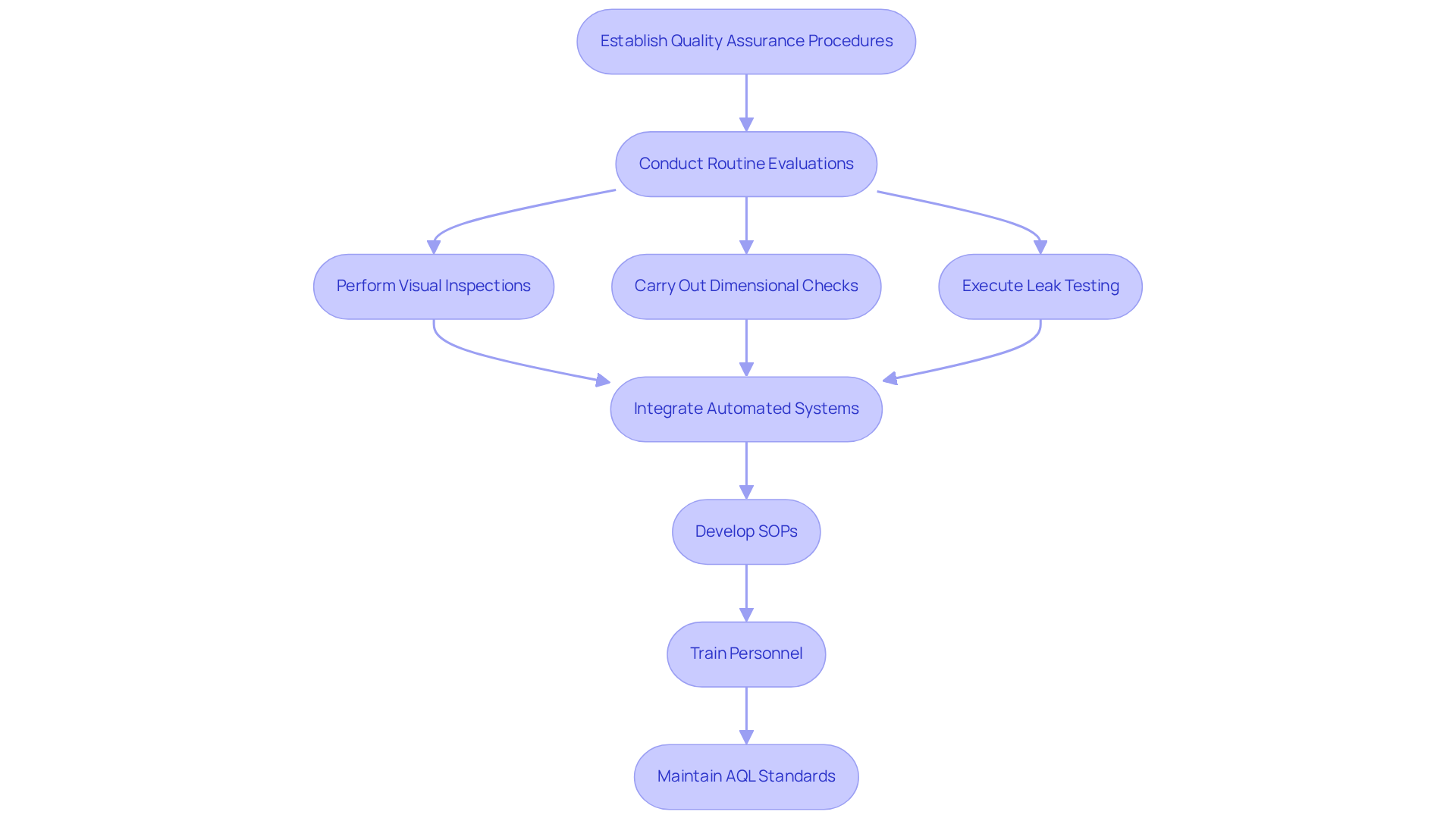
Establishing robust assurance procedures is essential for ensuring that the packaging for tablets meets the highest standards. Routine evaluations and assessments throughout the wrapping process are critical. Techniques such as:
- Visual inspections
- Dimensional checks
- Leak testing
are vital in identifying potential defects early. The integration of automated systems for standards checks significantly enhances both accuracy and efficiency, facilitating real-time monitoring and swift adjustments. Furthermore, automation and data analysis can predict and prevent issues, further refining the packing process.
Developing Standard Operating Procedures (SOPs) for packaging for tablets ensures consistency and compliance with regulatory standards, which is crucial in the pharmaceutical sector. Additionally, training personnel on control procedures is imperative, as human errors can lead to significant challenges in packaging integrity. By prioritizing standard control, manufacturers can effectively reduce waste, avoid costly recalls, and ultimately enhance customer satisfaction.
It is noteworthy that critical defects can have an Acceptable Quality Limit (AQL) ranging from 0.1 to 0.65, highlighting the necessity of maintaining high-quality standards. Moreover, effective quality control measures directly influence brand image and customer trust, making it a strategic priority for manufacturers.
Utilize Innovative Packaging Solutions for Enhanced Appeal
Innovative packaging for tablets significantly enhances their marketability, with blister packs emerging as a favored option. These packs provide superior moisture and oxygen barriers, effectively prolonging shelf life. Moreover, user-friendly features such as easy-open designs and child-resistant options not only enhance consumer experience but also prioritize safety.
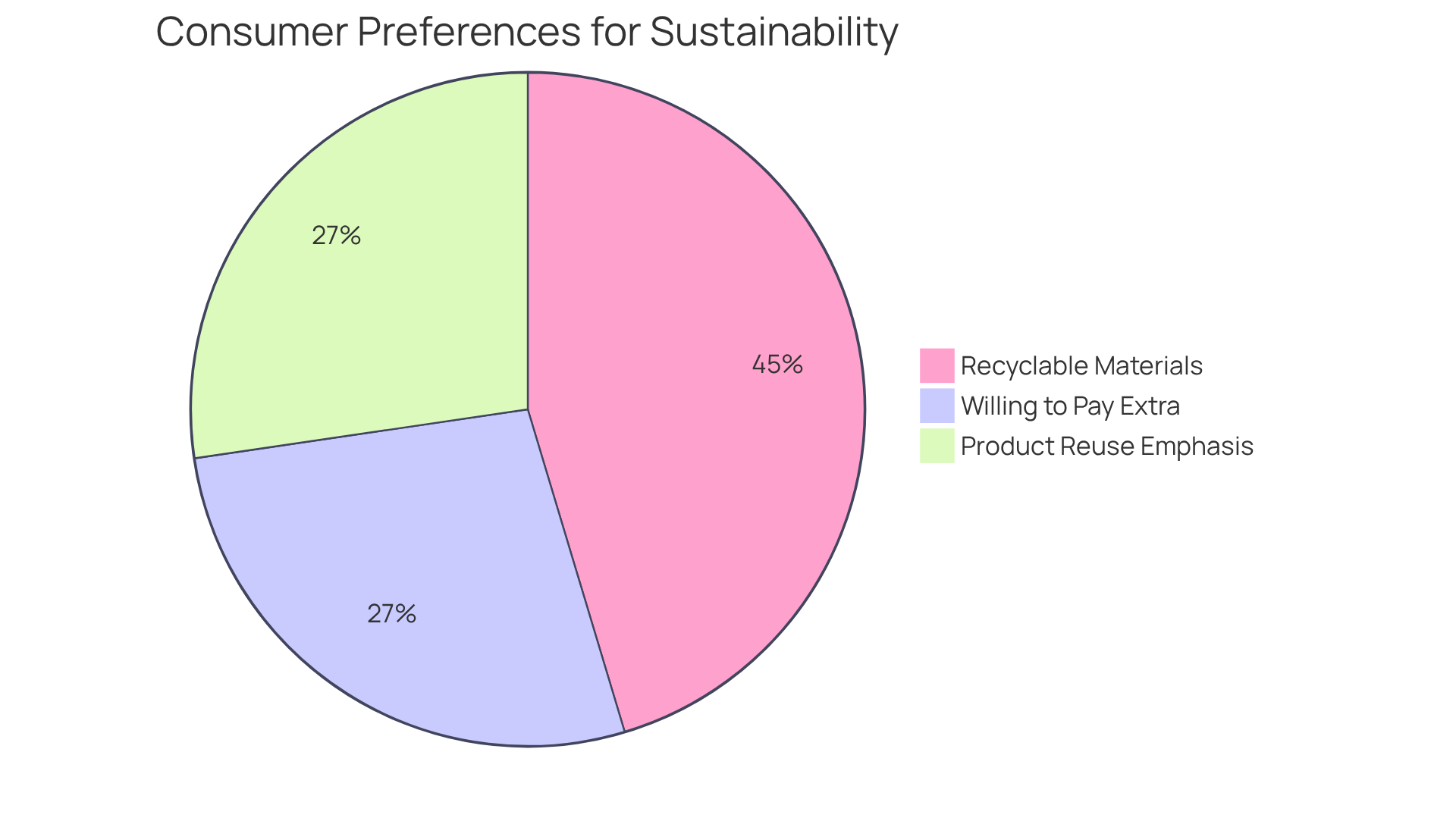
Recent statistics reveal that:
- 83% of consumers find recyclable materials important.
- Almost half are willing to pay extra for environmentally friendly items.
- 50% of consumers emphasize product reuse and waste minimization in their definitions of sustainability, underscoring the growing importance of sustainable practices.
This transition towards sustainability is evident in the increasing demand for biodegradable materials in containers. By investing in both creative and functional designs, manufacturers can attract a broader customer base while reinforcing their brand identity and commitment to quality.
Successful instances of blister pack designs illustrate how considerate packaging for tablets can enhance product attractiveness and meet changing consumer demands. As noted by Shorr Packaging, 'With almost three-quarters of consumers ready to switch to brands providing eco-friendly options, it’s evident that companies should prioritize these changing expectations.'
Furthermore, the sustainable packaging market is projected to grow from USD 292.71 billion in 2024 to USD 423.56 billion by 2029, underscoring the importance of adopting sustainable solutions in packaging.
Streamline Supply Chain Processes for Efficient Distribution
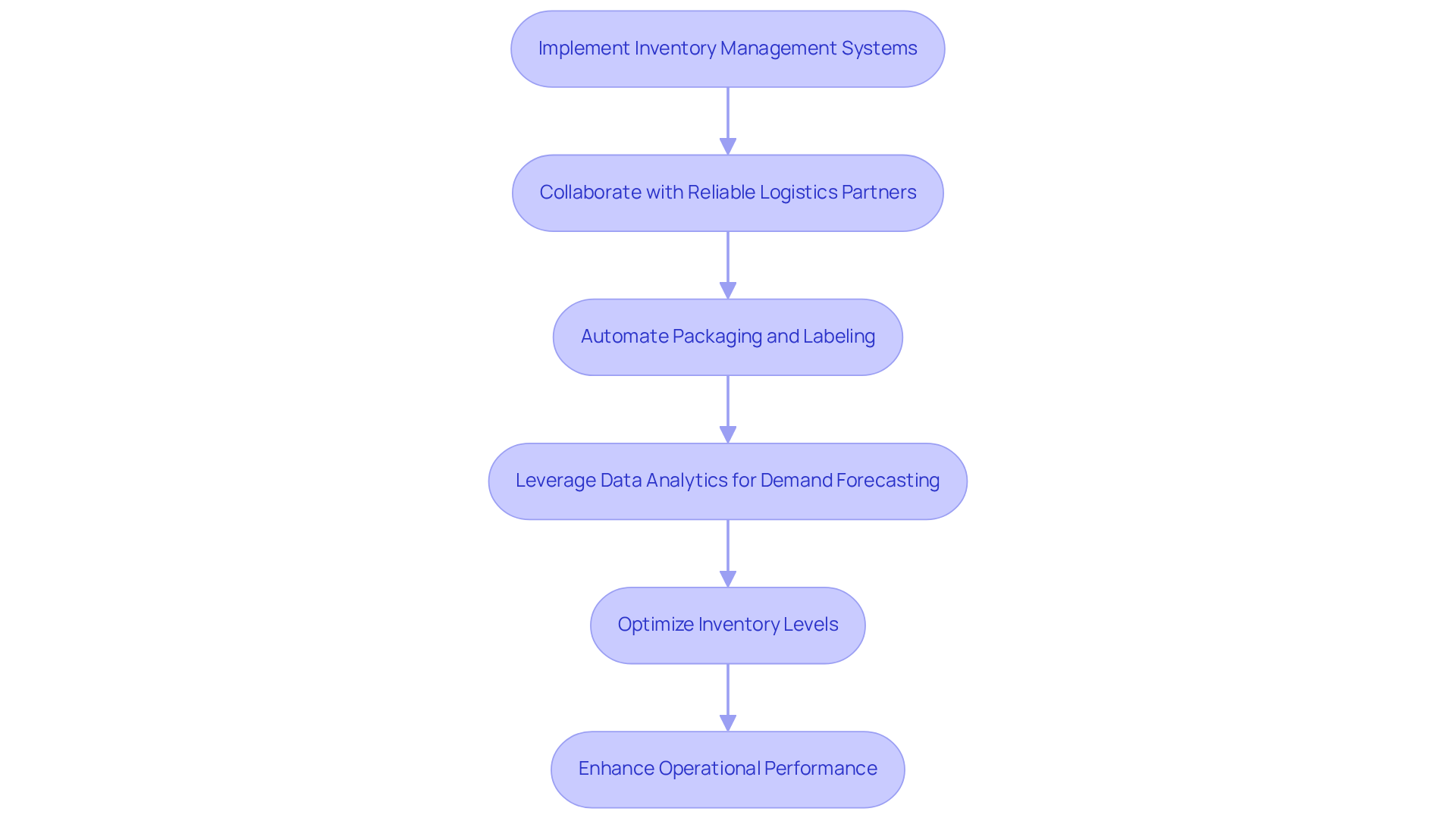
To achieve effective distribution of tablets, producers must enhance their supply chain procedures. Implementing advanced inventory management systems is crucial, as these systems provide real-time visibility into stock levels and order statuses, enabling proactive decision-making. Notably, 58% of retail brands and D2C manufacturers report inventory accuracy below 80%, underscoring the urgent need for effective inventory management.
Collaborating with reliable logistics partners further enhances distribution efficiency, ensuring timely delivery of products to retailers and consumers. As Wael Safwat aptly stated, "It’s not the organizations that are competing. It’s the supply chains that are competing." This statement emphasizes the critical role of logistics partnerships in maintaining a competitive advantage.
Additionally, automation in the packaging for tablets and labeling processes minimizes manual errors and accelerates production times, contributing to overall operational efficiency. Leveraging data analytics for demand forecasting allows manufacturers to optimize inventory levels, effectively reducing excess stock and minimizing waste.
With 74% of businesses experiencing delays in shipments, the importance of reliable logistics partners cannot be overstated. By prioritizing supply chain efficiency, companies can significantly enhance their operational performance and improve customer satisfaction.
Conclusion
Effective packaging for tablets transcends regulatory necessity; it is a strategic advantage that can profoundly influence market success. By prioritizing compliance, quality control, innovative design, and streamlined supply chain processes, manufacturers can ensure their products not only meet safety standards but also resonate with consumer expectations.
Key practices include:
- A thorough understanding of the regulatory landscape
- The implementation of robust quality control measures
- The utilization of innovative and sustainable packaging solutions
- The enhancement of supply chain efficiency
Each of these elements is crucial in safeguarding product integrity, enhancing consumer safety, and ultimately driving brand loyalty. The shift towards sustainable packaging reflects evolving consumer values, making it imperative for manufacturers to adapt and innovate.
In conclusion, the future of tablet packaging hinges on a holistic approach that integrates compliance, quality assurance, innovative design, and efficient logistics. As the market continues to evolve, embracing these best practices will not only enhance operational performance but also position brands as leaders in a competitive landscape. Taking proactive steps now can yield significant long-term benefits, ensuring that products are compliant, appealing, and accessible to consumers.
Frequently Asked Questions
What are the key regulatory requirements for tablet packaging?
Key regulatory requirements for tablet packaging include FDA guidelines for labeling and containers, ensuring materials are safe, effective, and not misleading. This includes the use of tamper-evident seals for over-the-counter medications.
What essential details must be included on tablet packaging?
Essential details that must be included on tablet packaging are the drug name, dosage instructions, active ingredients, batch number, expiry date, and manufacturer information.
Why is compliance important in tablet packaging?
Compliance is important in tablet packaging to ensure patient safety and avoid costly recalls and damage to brand reputation. Non-compliance can lead to serious consequences for manufacturers.
How can manufacturers ensure compliance with regulatory standards?
Manufacturers can ensure compliance by conducting thorough research on local and international regulations, implementing quality control measures, performing routine audits, and consulting with regulatory specialists.
What role does technology play in regulatory compliance for tablet packaging?
Technology can enhance container functionality and safety, providing a more comprehensive approach to meeting regulatory requirements in tablet packaging.
What are the consequences of non-compliance in tablet packaging?
Consequences of non-compliance can include costly recalls, damage to brand reputation, and potential legal issues, all of which can significantly impact a manufacturer's market presence.




