Overview
Key practices for collaborating with custom powder manufacturers are critical for achieving optimal results.
- Evaluate their experience and expertise to ensure they possess the necessary knowledge in the industry.
- Ensure adherence to quality certifications, which is essential for maintaining high standards of safety and reliability.
- Assess their technology and customization capabilities, as these factors significantly influence the quality of the final product.
- Examine the reliability of their supply chain to guarantee timely and consistent delivery.
By implementing these practices, businesses can select manufacturers that not only meet stringent quality and safety standards but also align with their specific operational needs. This strategic approach ultimately leads to enhanced product outcomes and increased customer satisfaction.
Introduction
Selecting the right custom powder manufacturer is a critical decision that can significantly impact product quality and business success. In a booming nutraceutical industry projected to reach $40 billion, understanding the nuances of this partnership is more important than ever.
What key practices ensure a fruitful collaboration while navigating quality control, regulatory compliance, and effective communication? This article delves into essential strategies that businesses can adopt to maximize their relationships with custom powder manufacturers, ultimately leading to enhanced product integrity and customer satisfaction.
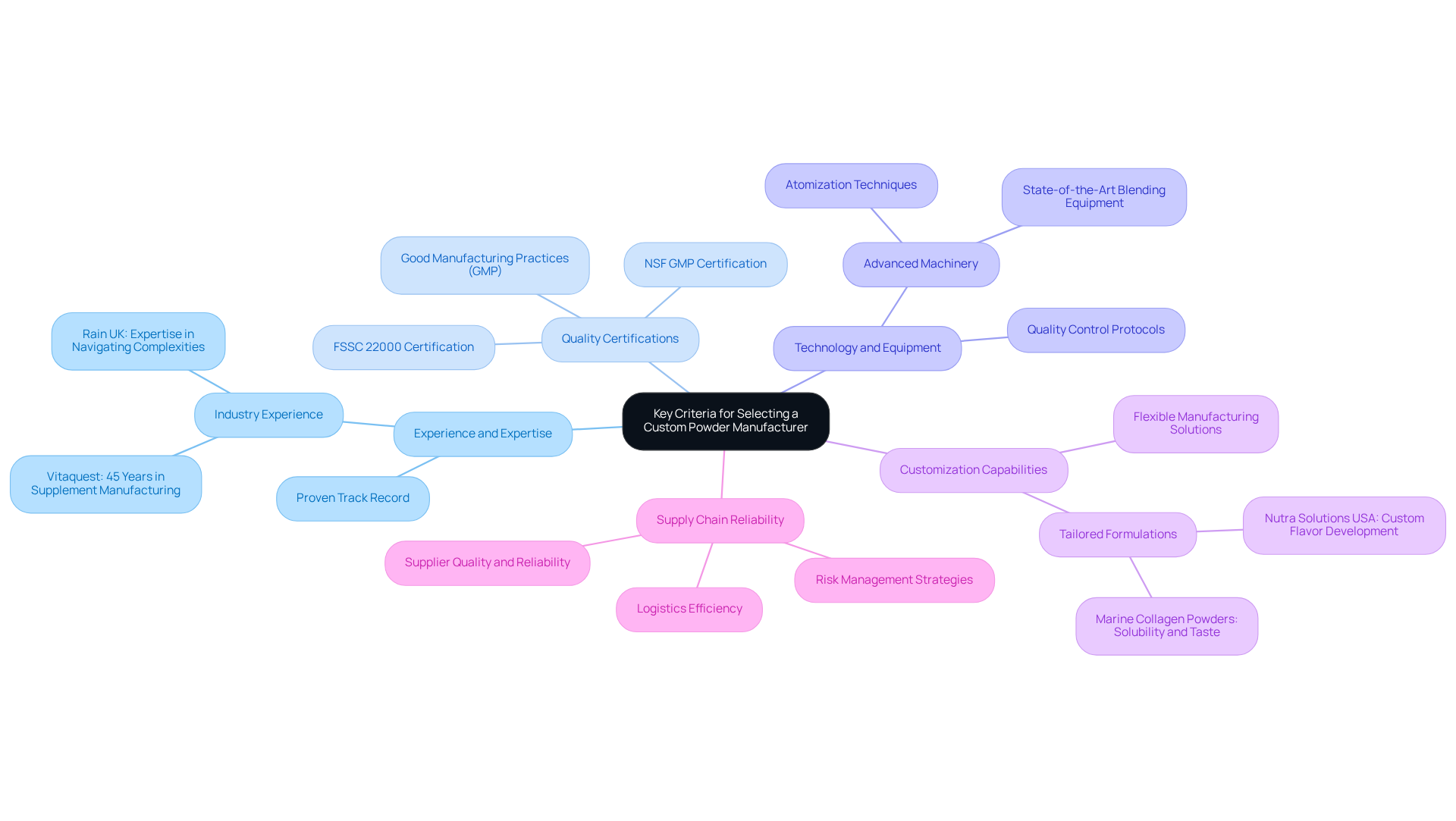
Identify Key Criteria for Selecting a Custom Powder Manufacturer
When selecting a custom powder manufacturer, it is essential to consider the following key criteria:
-
Experience and Expertise: Prioritize producers with a proven track record in creating the specific type of powder you require. For instance, Vitaquest International boasts over 45 years of expertise in the supplement manufacturing sector, significantly impacting the standard and effectiveness of the final offering, thereby ensuring it meets market requirements.
-
Quality Certifications: Verify that the producer adheres to Good Manufacturing Practices (GMP) and possesses relevant certifications such as NSF GMP certification or FSSC 22000. This adherence is crucial for maintaining strict standards throughout the manufacturing process, thereby protecting product integrity and consumer safety.
-
Technology and Equipment: Evaluate the technology and equipment employed by the producer. Advanced machinery, including techniques like atomization, can enhance production efficiency, consistency, and overall quality, all of which are vital in a competitive market.
-
Customization Capabilities: Assess the producer's capacity to meet your unique formulation needs, including flavoring, sweetening, and other tailored requirements. For example, Nutra Solutions USA specializes in customizing formulations to align with target audiences and goals, demonstrating flexibility in meeting client needs.
-
Supply Chain Reliability: Examine the producer's supply chain procedures to ensure prompt delivery of raw materials and finished goods. Key elements of supply chain management, such as logistics efficiency and risk management strategies, are essential for minimizing disruptions and ensuring consistent availability of products.
By concentrating on these criteria, companies can select custom powder manufacturers that meet their standards of excellence and operational requirements, ultimately leading to improved outcomes and heightened customer satisfaction.
Implement Robust Quality Control Measures in Powder Manufacturing
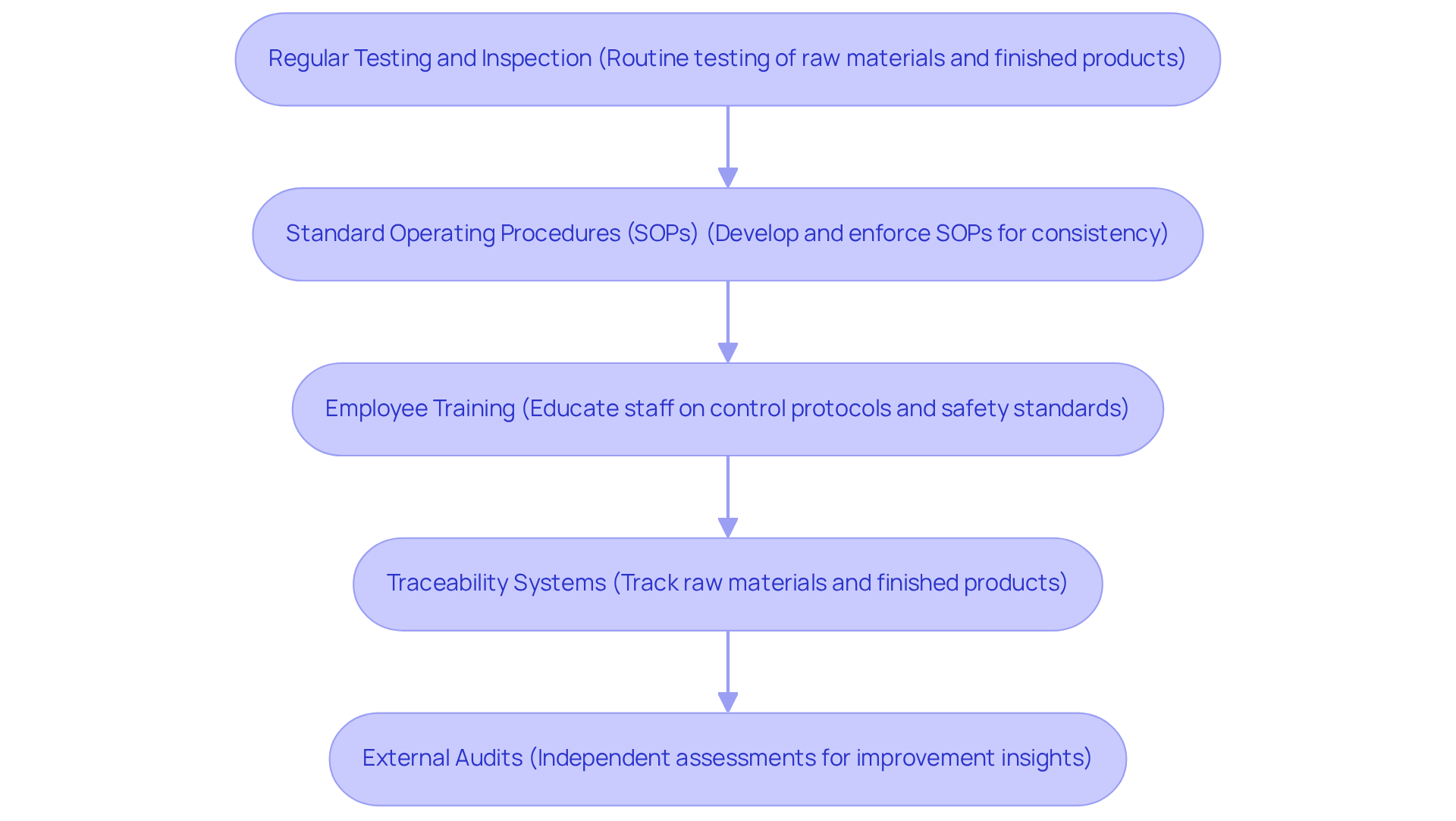
To implement robust quality control measures in powder manufacturing, consider the following practices:
-
Regular Testing and Inspection: Conduct routine testing of raw materials and finished products to ensure they meet specified standards of excellence. This includes physical, chemical, and microbiological testing. As Behzad Esmaeilian observes, "Statistical process control utilizes control charts to oversee the output of manufacturing processes, detect unnatural variations in the measurements, and determine their assignable causes."
-
Standard Operating Procedures (SOPs): Develop and enforce SOPs for all manufacturing processes. This ensures consistency and minimizes the risk of errors during production.
-
Employee Training: Consistently educate staff on control protocols and the significance of following safety standards. A well-informed workforce is crucial for maintaining high-quality production.
-
Traceability Systems: Implement traceability systems to track raw materials and finished products throughout the supply chain. This enables rapid identification and resolution of issues related to standards.
-
External Audits: Involve external auditors to assess your control processes. Independent assessments can provide valuable insights and help identify areas for improvement.
By implementing these standards of oversight, producers can improve the reliability of items and customer confidence, ultimately resulting in increased commercial success. Furthermore, it is essential to recognize that Six Sigma methodology aims for no more than 3.4 defects per million opportunities, emphasizing the importance of rigorous quality control practices. Integrating contemporary trends in testing and inspection for powder items will also guarantee that manufacturers remain ahead in a competitive environment.
Understand Regulatory Compliance Requirements for Nutraceuticals
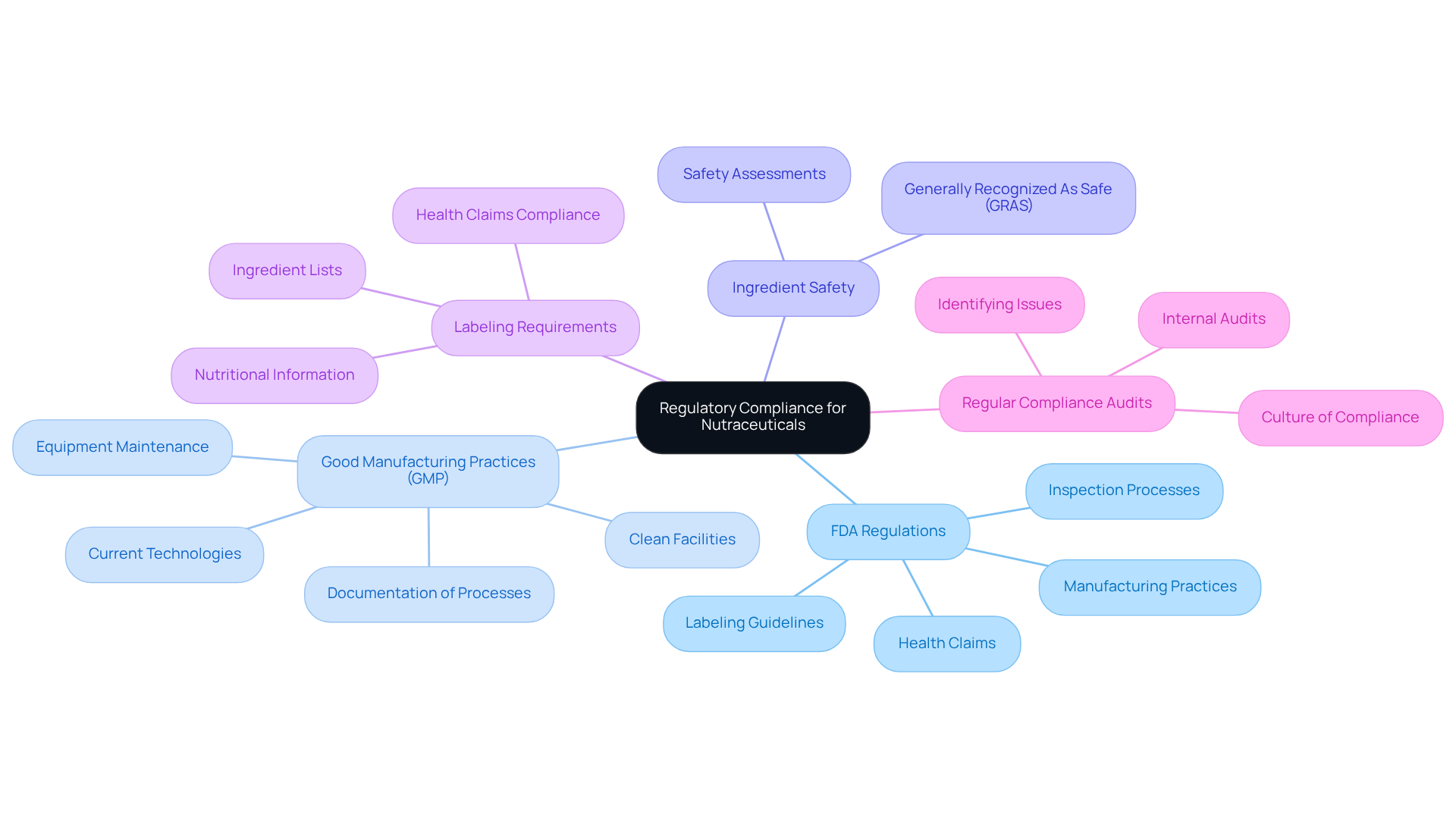
Navigating regulatory compliance in the nutraceutical industry necessitates a comprehensive understanding of several essential requirements:
-
FDA Regulations: Familiarity with the FDA's guidelines for dietary supplements is crucial. These guidelines encompass labeling, health claims, and manufacturing practices. Compliance with these regulations is not merely advisable; it is mandatory for market entry. The FDA's commitment to consumer safety is evident in its rigorous inspection processes, ensuring that most companies are found fully compliant with CGMP regulations. Non-compliance can lead to substantial repercussions, including recalls and legal actions.
-
Good Manufacturing Practices (GMP): Adhering to GMP standards is vital for ensuring the quality and safety of products. This includes maintaining clean facilities, proper equipment maintenance, and meticulous documentation of production processes. The 'C' in CGMP stands for 'current,' highlighting the necessity for manufacturers to employ up-to-date technologies and systems to meet regulatory expectations. As FDA Commissioner Dr. Gottlieb stated, "One of my top goals is ensuring that we achieve the right balance between preserving consumers’ access to lawful supplements while still upholding our solemn obligation to protect the public from unsafe and illegal items."
-
Ingredient Safety: Conducting thorough safety assessments for all ingredients is essential. This process involves reviewing scientific literature and confirming that ingredients are Generally Recognized As Safe (GRAS). Such diligence not only protects consumers but also enhances the credibility of your offerings in a competitive market.
-
Labeling Requirements: Adherence to labeling regulations is critical. These regulations dictate the necessary information on packaging, including ingredient lists, nutritional information, and health claims. Accurate labeling fosters consumer trust and aligns with FDA expectations.
-
Regular Compliance Audits: Implementing regular internal audits serves as a proactive strategy to evaluate compliance with regulatory requirements. This practice helps identify potential issues before they escalate, ensuring that operations remain aligned with industry standards. Regular audits can uncover areas for improvement and contribute to a culture of compliance within the organization.
By understanding and adhering to these regulatory requirements, producers can ensure product safety and maintain consumer confidence, which is vital for sustained success in the nutraceutical industry. The dietary supplement industry has experienced significant growth, with its current market size reaching $40 billion, up from $4 billion since the passage of the Dietary Supplement Health and Education Act (DSHEA) in 1994. This growth underscores the increasing importance of compliance in a rapidly evolving industry.
Foster Effective Communication and Collaboration with Manufacturers
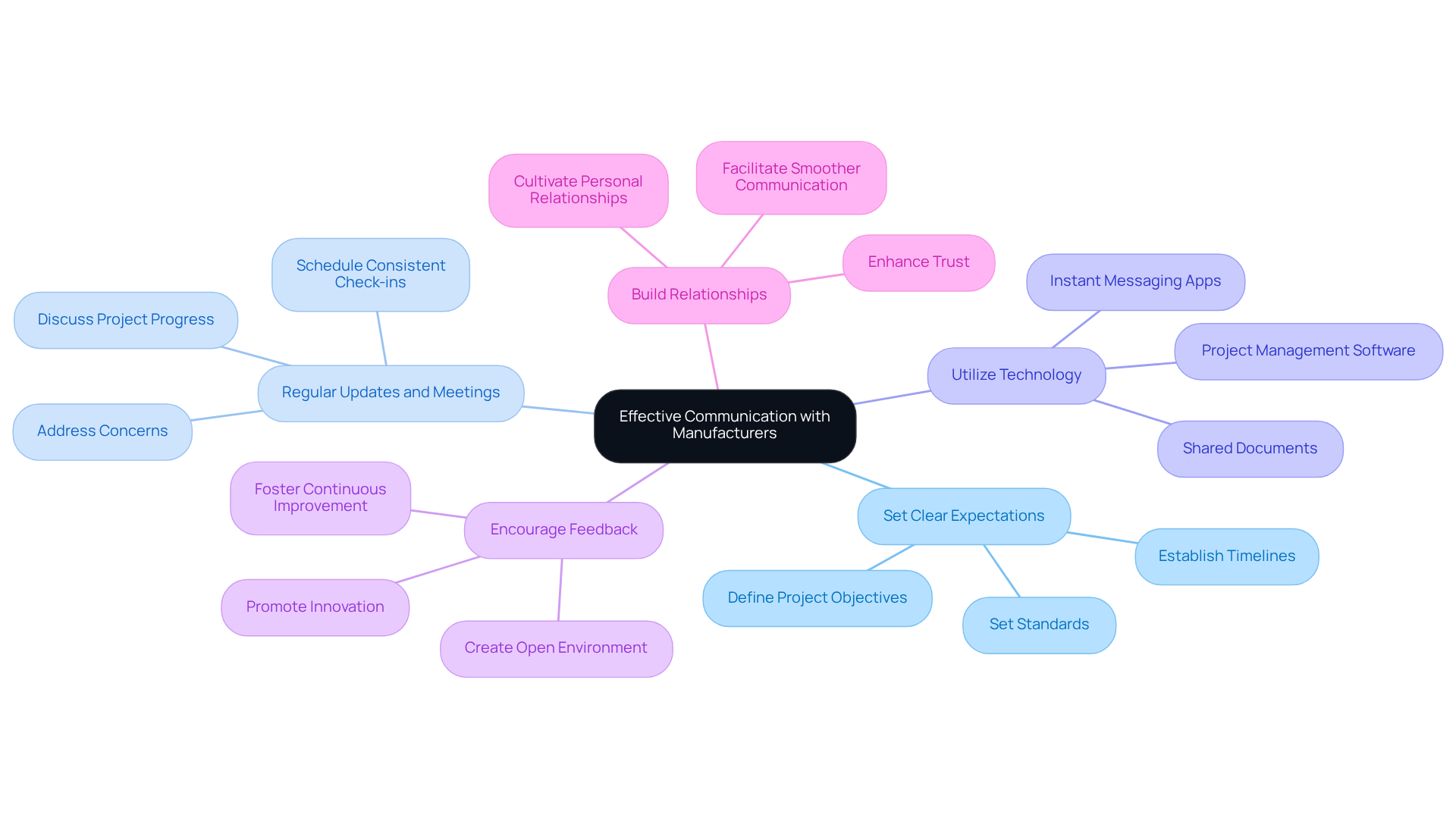
To foster effective communication and collaboration with custom powder manufacturers, implement the following strategies:
-
Set Clear Expectations: Clearly define project objectives, timelines, and standards from the outset. This alignment reduces the risk of misunderstandings and enhances operational efficiency.
-
Regular Updates and Meetings: Schedule consistent check-ins and updates to discuss project progress, address concerns, and make necessary adjustments. Keeping everyone informed and engaged is crucial for success.
-
Utilize Technology: Leverage communication tools and platforms that facilitate real-time collaboration. This includes project management software, shared documents, and instant messaging apps which streamline processes.
-
Encourage Feedback: Create an open environment where both parties can offer input on processes and items. Constructive feedback fosters continuous improvement and innovation.
-
Build Relationships: Invest time in cultivating personal relationships with key contacts at the manufacturing facility. Strong relationships enhance trust and facilitate smoother communication.
By implementing these strategies, businesses can significantly enhance collaboration with manufacturers, leading to improved product quality and operational efficiency.
Conclusion
Selecting the right custom powder manufacturer is not merely a task; it is a critical undertaking that can significantly influence product quality and business success. By concentrating on essential criteria such as experience, quality certifications, advanced technology, customization capabilities, and supply chain reliability, businesses can ensure they partner with manufacturers that align with their operational goals and standards of excellence.
The article underscores several best practices, including:
- The implementation of robust quality control measures
- Understanding regulatory compliance requirements
- Fostering effective communication and collaboration with manufacturers
Regular testing, adherence to GMP, and maintaining transparency through regular updates are pivotal for ensuring product integrity and consumer safety. Furthermore, establishing strong relationships with manufacturers is essential for facilitating smoother operations and enhancing overall product quality.
In a competitive market, prioritizing these practices not only enhances product reliability but also builds consumer trust. As the nutraceutical industry continues to expand, staying informed about compliance and quality assurance will be paramount for success. Embracing these key practices can lead to improved outcomes and ultimately contribute to a thriving business in the custom powder manufacturing landscape.
Frequently Asked Questions
What key criteria should be considered when selecting a custom powder manufacturer?
The key criteria include experience and expertise, quality certifications, technology and equipment, customization capabilities, and supply chain reliability.
Why is experience and expertise important in selecting a custom powder manufacturer?
Experience and expertise are crucial because they indicate a producer's proven track record in creating the specific type of powder required, which impacts the standard and effectiveness of the final product.
What quality certifications should a custom powder manufacturer have?
A custom powder manufacturer should adhere to Good Manufacturing Practices (GMP) and possess relevant certifications such as NSF GMP certification or FSSC 22000 to ensure strict standards and consumer safety.
How does technology and equipment influence the selection of a custom powder manufacturer?
Advanced technology and equipment, such as atomization techniques, enhance production efficiency, consistency, and overall quality, which are vital for maintaining competitiveness in the market.
What are customization capabilities in the context of custom powder manufacturing?
Customization capabilities refer to a producer's ability to meet unique formulation needs, including aspects like flavoring and sweetening, to align with specific target audiences and goals.
Why is supply chain reliability important when choosing a custom powder manufacturer?
Supply chain reliability is important because it ensures the prompt delivery of raw materials and finished goods, minimizing disruptions and ensuring consistent product availability.




