Overview
The four key strategies for effective warehouse assembly in nutraceuticals are essential for enhancing operational efficiency and product safety:
- Establishing clear workflows is crucial; it ensures that all processes are streamlined and easily understood.
- Ensuring regulatory compliance is non-negotiable, as adherence to safety and regulatory standards protects both the company and its consumers.
- Implementing robust quality control practices serves as a proactive measure to maintain product integrity.
- Optimizing inventory management is vital for maintaining efficient stock control systems.
Together, these strategies form a comprehensive approach that not only improves operational efficiency but also reinforces product safety within the nutraceutical sector.
Introduction
The intricacies of warehouse assembly in the nutraceutical industry necessitate a profound understanding of operational efficiency and regulatory compliance. As this sector continues to expand, the demand for effective strategies intensifies—strategies that not only streamline processes but also uphold safety and quality in product handling. Yet, companies face increasing challenges, including labor shortages and the complexities of compliance regulations.
How can organizations optimize their warehouse assembly operations to meet the surging consumer demands while maintaining high standards? This article explores four pivotal strategies designed to transform warehouse assembly practices, thereby enhancing both productivity and compliance within the nutraceutical landscape.
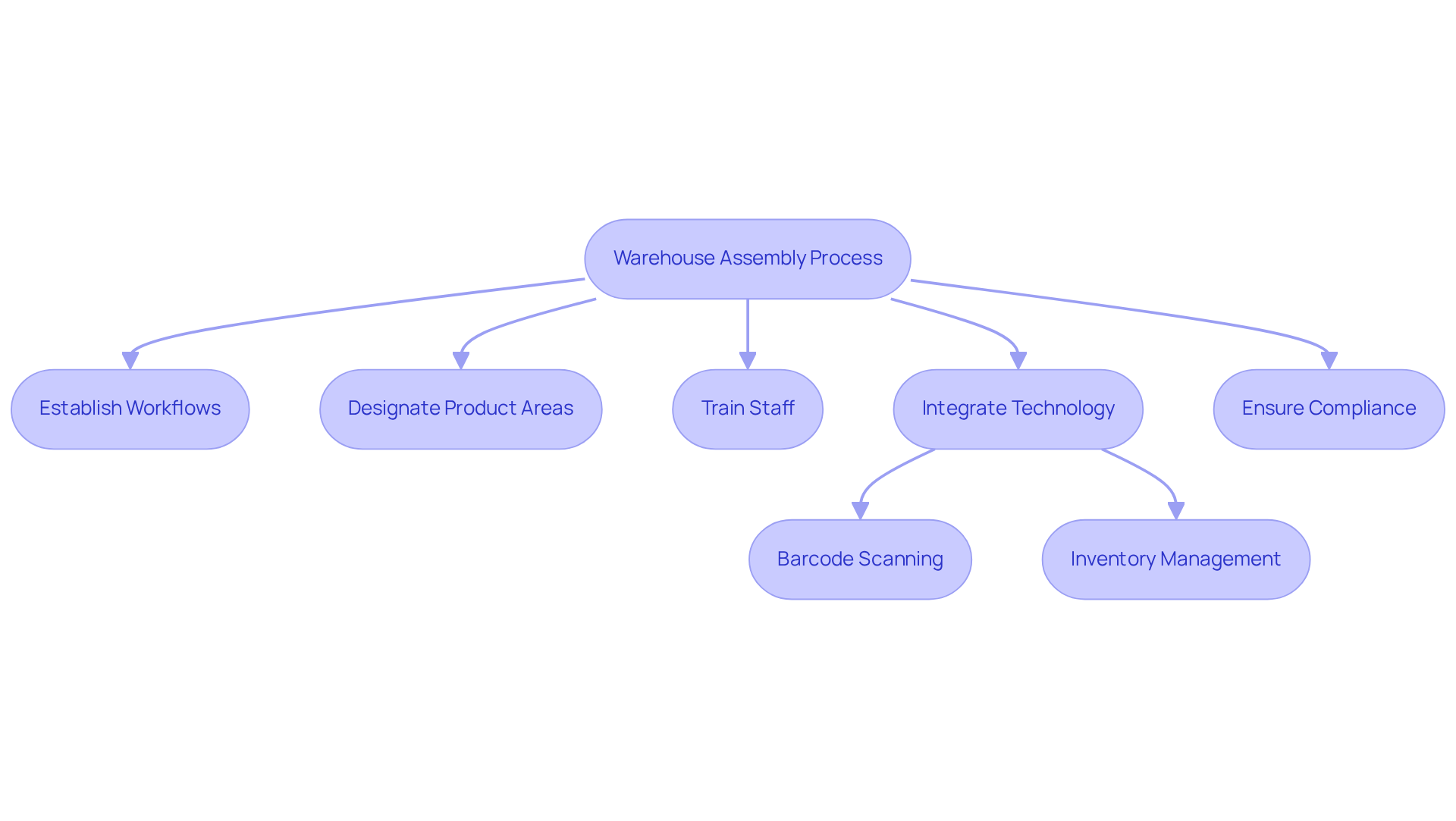
Understand Warehouse Assembly Fundamentals in Nutraceuticals
The process of warehouse assembly in the nutraceutical sector is complex and requires meticulous attention to detail, especially because of the sensitivity of the items involved. Strict adherence to safety protocols is paramount, as it ensures the integrity and compliance with regulatory standards. For example, nutraceuticals often mandate temperature-controlled environments and tamper-proof packaging to guarantee safety and efficacy. Compliance with these standards is crucial, as it mitigates risks related to liability and safety.
Implementing a structured process for warehouse assembly is essential for enhancing operational efficiency. This involves:
- Establishing clear workflows
- Designating specific areas for various types of products
- Providing comprehensive training for staff
The use of specialized production lines tailored for specific product categories can significantly reduce errors and improve processing speed. Moreover, with 73% of warehouse operators facing challenges in attracting qualified workers, the establishment of organized systems becomes increasingly vital.
The integration of technology is critical in optimizing warehouse assembly operations. The adoption of barcode scanning and advanced inventory management systems enhances the manufacturing process, facilitating precise monitoring of items from receipt to shipment. Statistics reveal that companies leveraging such technologies can enhance order accuracy and decrease fulfillment times, which is essential in meeting the rising consumer demand for nutraceuticals. Furthermore, the warehouse automation sector is projected to grow from $21.42 billion in 2024 to $24.09 billion by 2025, underscoring the significance of technology in improving warehouse operations. This foundational understanding is vital for refining warehouse construction processes in the nutraceutical industry, ensuring that companies can adeptly manage the complexities of item handling and distribution.
Ensure Regulatory Compliance in Assembly Operations
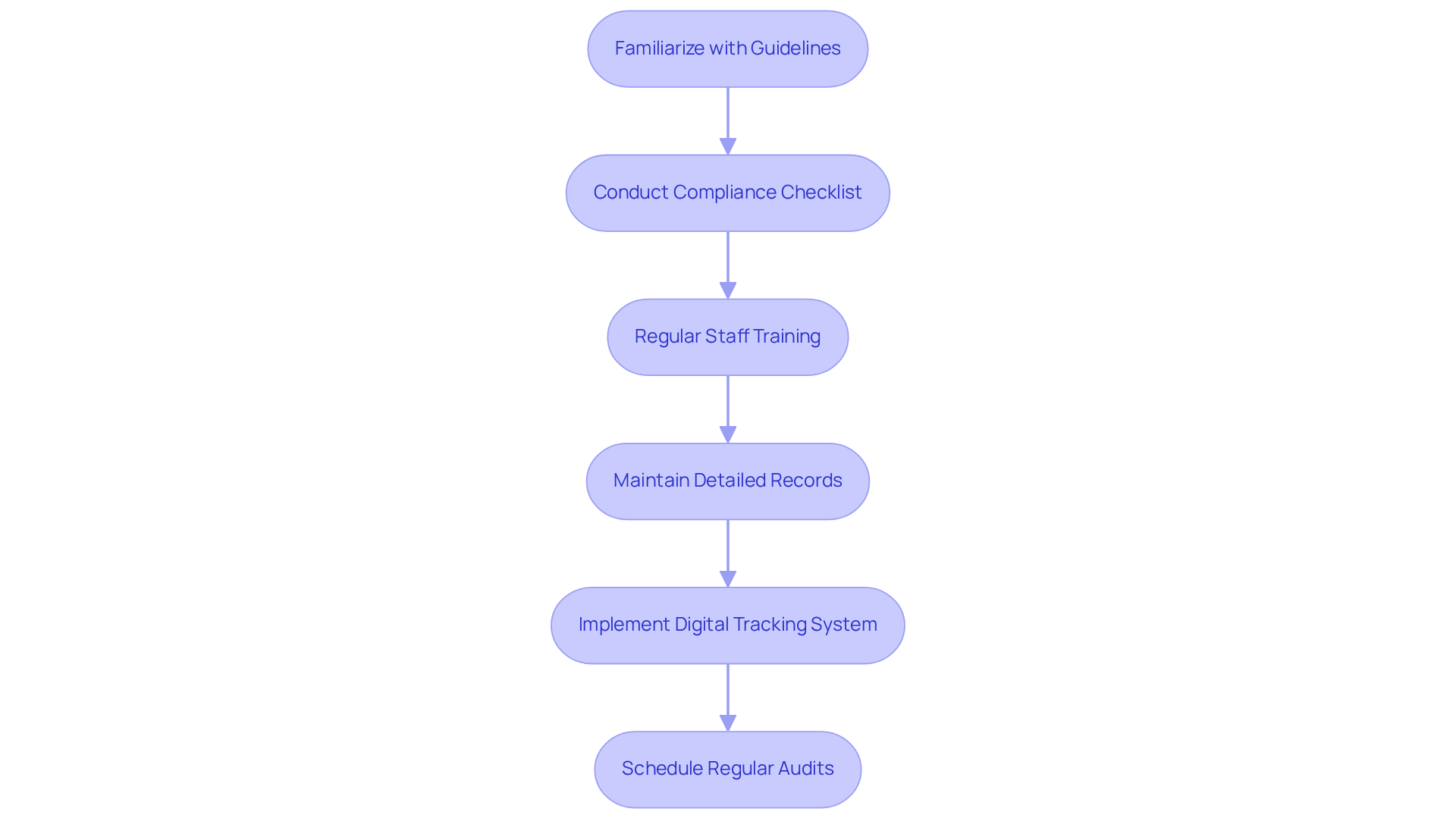
To ensure regulatory compliance in production operations, it is vital to familiarize yourself with the relevant guidelines set forth by organizations such as the FDA, particularly the regulation 21 CFR Part 117, which establishes cGMP regulations as part of the Current Good Manufacturing Practice for Human Food rule. Adhering to Current Good Manufacturing Practices (cGMP) is crucial, as these regulations dictate how products should be manufactured, packaged, and labeled.
Executing a thorough compliance checklist that encompasses all facets of the construction can greatly assist in upholding these standards. Regular training sessions for staff on compliance requirements and updates to regulations are also essential to ensure ongoing adherence.
Furthermore, keeping detailed records of all manufacturing procedures, including batch documentation and quality assurance evaluations, is essential for demonstrating adherence during inspections. Non-compliance can result in serious repercussions, including recalls, penalties, and harm to the company's reputation.
For instance, a nutraceutical firm could introduce a digital tracking system that records every stage of manufacturing, guaranteeing that all items comply with regulatory standards prior to their entry into the market. Additionally, cGMP certification typically lasts for one to three years, emphasizing the need for regular audits and continuous adherence to these standards. This proactive approach not only enhances compliance but also builds consumer trust and safeguards the company's reputation.
Implement Robust Quality Control Practices
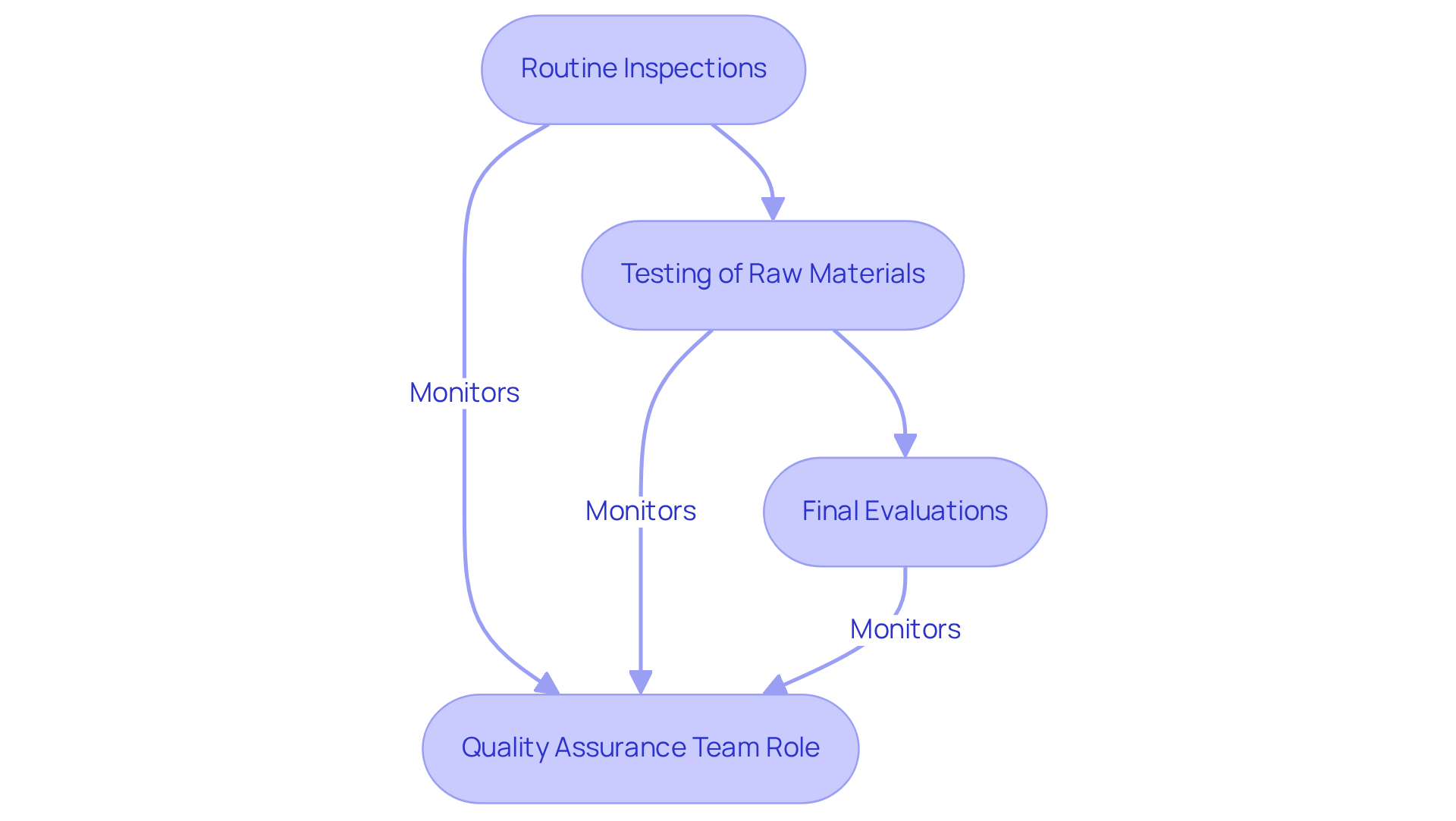
Establishing robust quality control measures is imperative for any organization aiming to maintain high standards. This process involves essential steps, including:
- Routine inspections of the manufacturing process
- Thorough testing of raw materials
- Comprehensive final evaluations
A dedicated quality assurance team plays a crucial role in monitoring compliance with these standards, proactively identifying potential issues before they escalate. For instance, conducting regular assessments of the warehouse assembly guarantees that all machinery operates optimally and that products are assembled according to established standards.
Furthermore, leveraging third-party testing services to validate the quality of finished products significantly enhances credibility. Organizations should also adopt a continuous improvement strategy, utilizing insights gleaned from quality control procedures to refine production practices. This proactive approach not only ensures compliance but also fosters consumer confidence in the brand. By prioritizing quality, businesses can position themselves as leaders in the packaging and logistics industry, ultimately driving success.
Optimize Inventory Management for Seamless Assembly
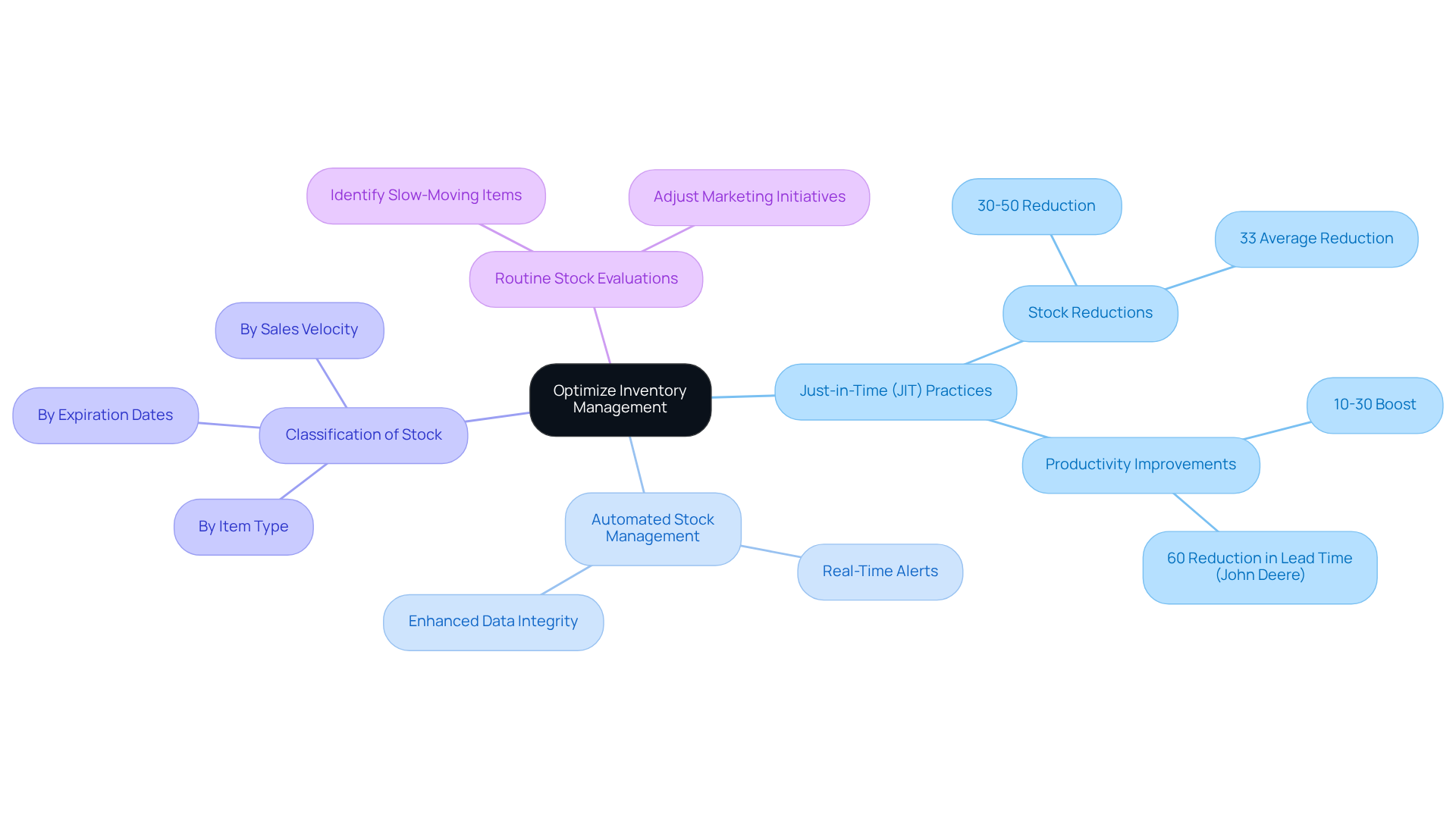
Efficient stock control is essential for enhancing production processes in the nutraceutical industry. Adopting Just-in-Time (JIT) stock practices enables companies to maintain minimal levels of supplies, thereby decreasing surplus stock and waste. An automated stock management system, for instance, can alert personnel when supply levels fall below a set limit, facilitating prompt reordering and ensuring that production processes remain uninterrupted by material shortages.
Classifying stock by item type, expiration dates, and sales velocity significantly enhances organization and accessibility, allowing teams to quickly locate essential materials. Routine stock evaluations are crucial for identifying slow-moving items that may require marketing initiatives or adjustments in production procedures. By streamlining these stock management practices, nutraceutical companies can markedly improve warehouse assembly efficiency, respond swiftly to market demands, and ultimately enhance customer satisfaction.
Statistical evidence underscores the effectiveness of JIT practices; companies that successfully implement these principles can achieve stock reductions of 30-50% while boosting productivity by 10-30%. According to Aberdeen Group, manufacturers that adopt JIT principles have reduced stock levels by an average of 33% while simultaneously enhancing service levels by 8.5%. This integrated approach not only optimizes stock levels but also aligns with the increasing trend of advanced data analytics in stock management, which is increasingly recognized as vital for gaining a competitive edge in the industry. Moreover, companies like John Deere have showcased the impact of JIT, achieving an 85% reduction in inventory along with significant cost savings. Furthermore, 85% of manufacturing executives believe that advanced JIT implementations leveraging these technologies will be crucial for maintaining competitive advantage within the next five years.
Conclusion
Understanding the complexities of warehouse assembly in the nutraceutical sector is crucial for ensuring efficiency, compliance, and quality. The strategies outlined in this article emphasize the importance of structured processes, regulatory adherence, robust quality control, and optimized inventory management. By implementing these best practices, companies can navigate the unique challenges posed by the nutraceutical industry and enhance their operational effectiveness.
Key insights include:
- The necessity of establishing clear workflows and designated areas for various products.
- The critical role of technology in streamlining operations.
- Compliance with regulations such as cGMP, which is not only a legal obligation but also a means to build consumer trust.
- Prioritizing quality control through regular inspections and third-party testing to foster reliability and credibility within the market.
- Efficient inventory management practices, particularly Just-in-Time strategies, which can significantly reduce waste and improve responsiveness to consumer demand.
Ultimately, the focus on effective warehouse assembly in nutraceuticals is not just about maintaining standards; it is about positioning companies for long-term success in a competitive landscape. By embracing these strategies, businesses can enhance their operational frameworks, ensure regulatory compliance, and deliver high-quality products that meet the evolving needs of consumers. Taking action to refine warehouse assembly processes will not only safeguard a company's reputation but also contribute to the overall growth and sustainability of the nutraceutical industry.
Frequently Asked Questions
What is the importance of safety protocols in warehouse assembly for nutraceuticals?
Safety protocols are crucial in warehouse assembly for nutraceuticals as they ensure the integrity and compliance with regulatory standards. This includes maintaining temperature-controlled environments and using tamper-proof packaging to guarantee safety and efficacy, which mitigates risks related to liability and safety.
What are the key components of an effective warehouse assembly process in the nutraceutical sector?
An effective warehouse assembly process includes establishing clear workflows, designating specific areas for various types of products, and providing comprehensive training for staff.
How can specialized production lines benefit warehouse assembly operations?
Specialized production lines tailored for specific product categories can significantly reduce errors and improve processing speed, enhancing overall operational efficiency.
What challenges do warehouse operators face in the nutraceutical industry?
Approximately 73% of warehouse operators face challenges in attracting qualified workers, making the establishment of organized systems increasingly vital for successful operations.
How does technology play a role in optimizing warehouse assembly operations?
The integration of technology, such as barcode scanning and advanced inventory management systems, enhances the manufacturing process by facilitating precise monitoring of items from receipt to shipment, improving order accuracy and decreasing fulfillment times.
What is the projected growth of the warehouse automation sector, and why is it significant?
The warehouse automation sector is projected to grow from $21.42 billion in 2024 to $24.09 billion by 2025. This growth underscores the significance of technology in improving warehouse operations and meeting the rising consumer demand for nutraceuticals.
Why is a foundational understanding of warehouse assembly important in the nutraceutical industry?
A foundational understanding of warehouse assembly is vital for refining warehouse construction processes, ensuring that companies can adeptly manage the complexities of item handling and distribution in the nutraceutical industry.




