Overview
The four key strategies for medical packaging compliance are essential for navigating the complexities of the industry.
- Understanding regulatory requirements lays the foundation for adherence to standards.
- Implementing design strategies that enhance safety ensures that products are user-friendly and reduce risk.
- Establishing robust quality control measures is crucial for maintaining high standards throughout the packaging process.
- Fostering collaboration across the supply chain strengthens relationships and promotes transparency.
These strategies underscore the importance of regulatory adherence, proactive design for user safety, systematic quality assurance practices, and stakeholder engagement. By embracing these principles, organizations can effectively navigate the intricate compliance landscape and achieve lasting success.
Introduction
Navigating the complex landscape of medical packaging compliance is not merely a regulatory necessity; it is a critical factor that determines the safety and efficacy of healthcare products. As the stakes rise with evolving regulations from organizations such as the FDA and ISO, a comprehensive understanding of the intricate requirements becomes paramount for manufacturers. This article explores four essential strategies that ensure compliance while enhancing product integrity and user safety. With impending changes in regulations and the increasing demand for innovation, the pressing question remains: how can companies effectively balance compliance with creativity in their packaging solutions?
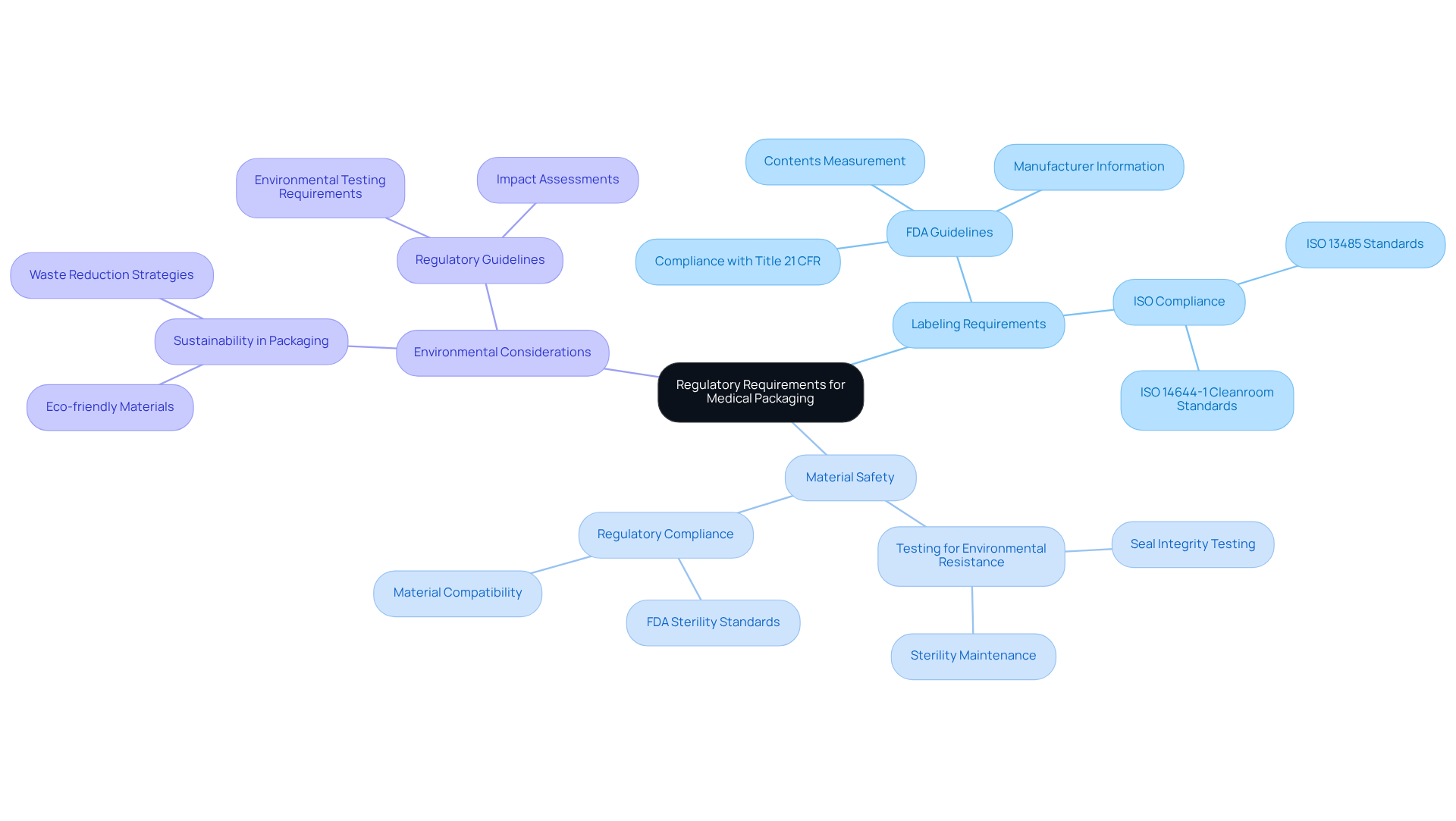
Understand Regulatory Requirements for Medical Packaging
To achieve adherence in medical containers, it is crucial to fully understand the regulations established by key organizations such as the FDA, EMA, and ISO standards. This includes a thorough understanding of:
- Labeling requirements
- Material safety
- Environmental considerations
Regularly reviewing updates from these regulatory bodies is essential for maintaining compliance. For instance, the FDA mandates that all medical packaging must effectively safeguard products from contamination and degradation throughout their lifecycle. In fiscal year 2023, the FDA issued 24 Warning Letters, underscoring the importance of adhering to these guidelines.
As Jason Haider, founder and CEO of Xenco Medical, states, "Due to the FDA's strict criteria for the sterility of medical devices it endorses, manufacturers must undergo comprehensive testing to demonstrate that the medical packaging can withstand environmental influences that could jeopardize the sterility of its contents."
Furthermore, the Quality Management System Regulation (QMSR) Final Rule will take effect on February 2, 2026, further emphasizing the necessity for compliance. Engaging with legal professionals or regulatory advisors can provide valuable insights into the specific criteria relevant to your products, ensuring that your containers meet all essential guidelines and regulations. Additionally, adopting a proactive, technology-driven approach to compliance can streamline the validation process and enhance overall efficiency.
Implement Design Strategies for Compliance
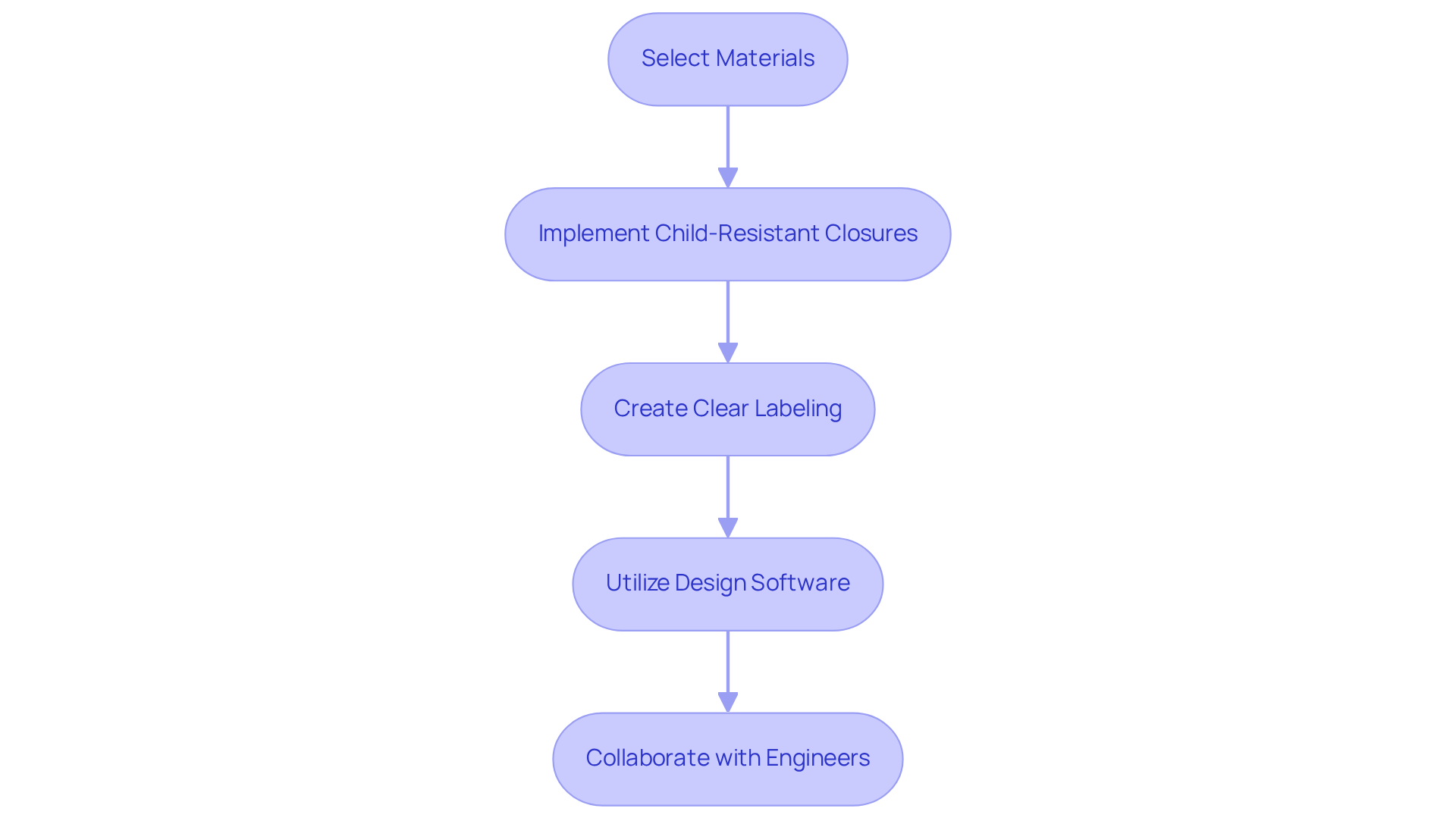
Creating medical packaging that is compliant necessitates a meticulous selection of materials that not only meet safety standards but also enhance user experience. Implementing child-resistant closures is not merely a best practice; it is a regulatory requirement for products intended for households with children, as mandated by the Poison Prevention Packaging Act (PPPA).
Moreover, clear labeling that includes dosage instructions and warnings significantly bolsters user safety, ensuring correct and safe product usage. Notably, statistics reveal that the adoption of child-resistant containers has resulted in a reduction of accidental poisoning-related child mortality by as much as 45%.
Utilizing advanced design software to simulate container performance can effectively identify potential compliance issues early in the design process. Collaboration with design engineers is crucial, as it ensures that the design meets both aesthetic and functional criteria, ultimately yielding a product that is both safe and appealing to consumers.
Insights from organizations such as the Consumer Product Safety Commission (CPSC) underscore the necessity of adhering to these regulations to safeguard children from potential hazards.
Establish Robust Quality Control Measures
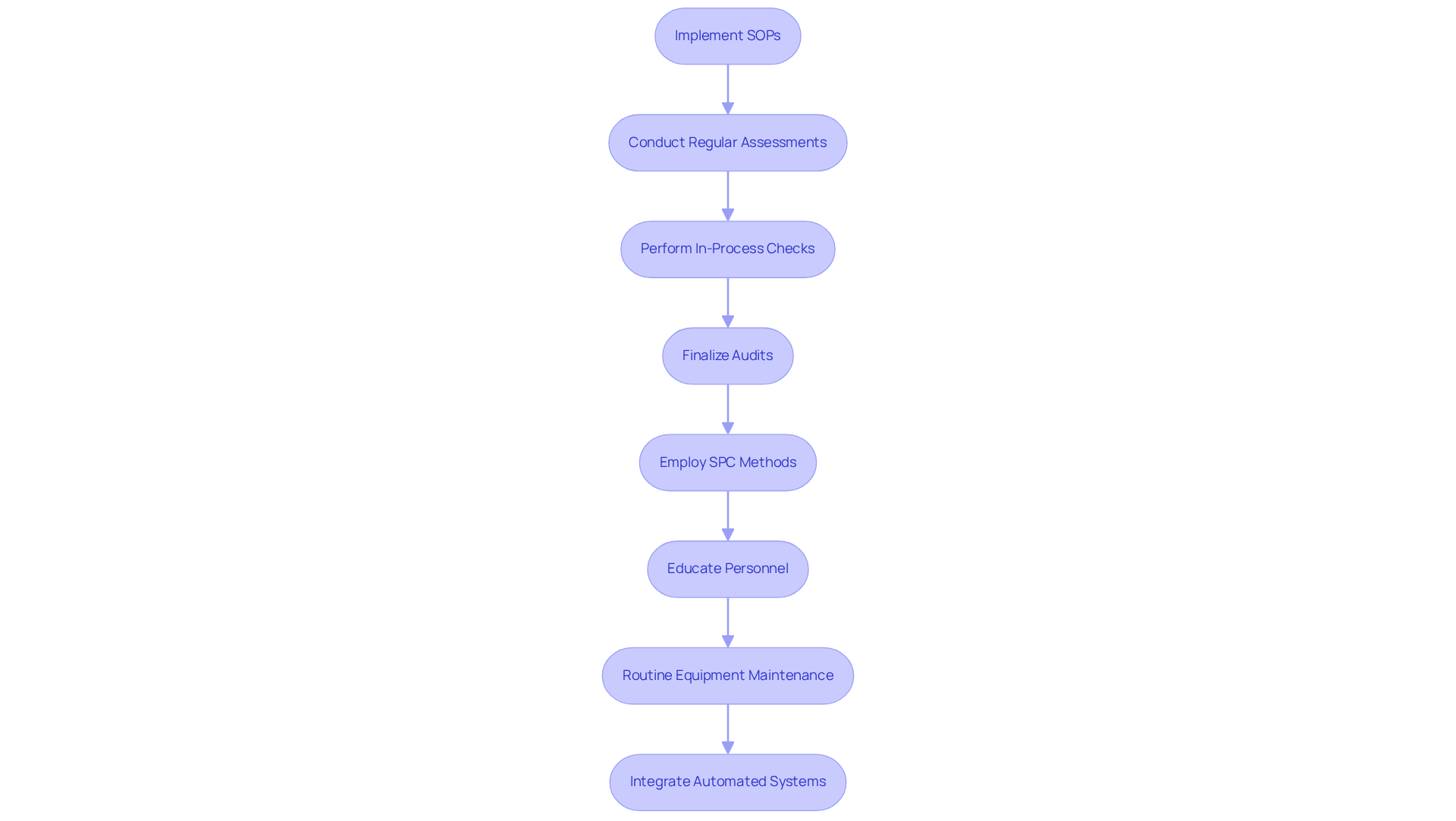
Implementing robust quality control practices is essential for maintaining high standards in the nutraceutical industry. This process begins with the execution of standard operating procedures (SOPs) at every stage of the wrapping process. Regular assessments of medical packaging materials, in-process checks during filling, and final audits before distribution form the backbone of these SOPs. By employing statistical process control (SPC) methods, companies can effectively monitor fluctuations in medical packaging standards, enabling timely interventions to address potential issues. Statistical analysis is pivotal in this context, as it underpins data-driven decision-making and helps in identifying trends that may indicate underlying problems.
Moreover, educating personnel on regulatory standards and performance expectations cultivates a culture of responsibility and excellence. Routine equipment maintenance and the integration of automated systems are also vital for enhancing the efficacy of control measures. For example, a nutraceutical producer that adopted a comprehensive management system and leveraged SPC techniques experienced a significant 30% reduction in medical packaging defects, markedly improving their compliance status. This underscores the critical role of SOPs not only in boosting product quality but also in ensuring adherence to regulatory mandates.
However, it is crucial to recognize potential challenges in implementing SOPs, such as resistance to change or insufficient training, which can compromise their effectiveness. In conclusion, establishing strong SOPs can lead to improved product integrity, heightened customer satisfaction, and a more robust regulatory position.
Foster Collaboration Across the Supply Chain
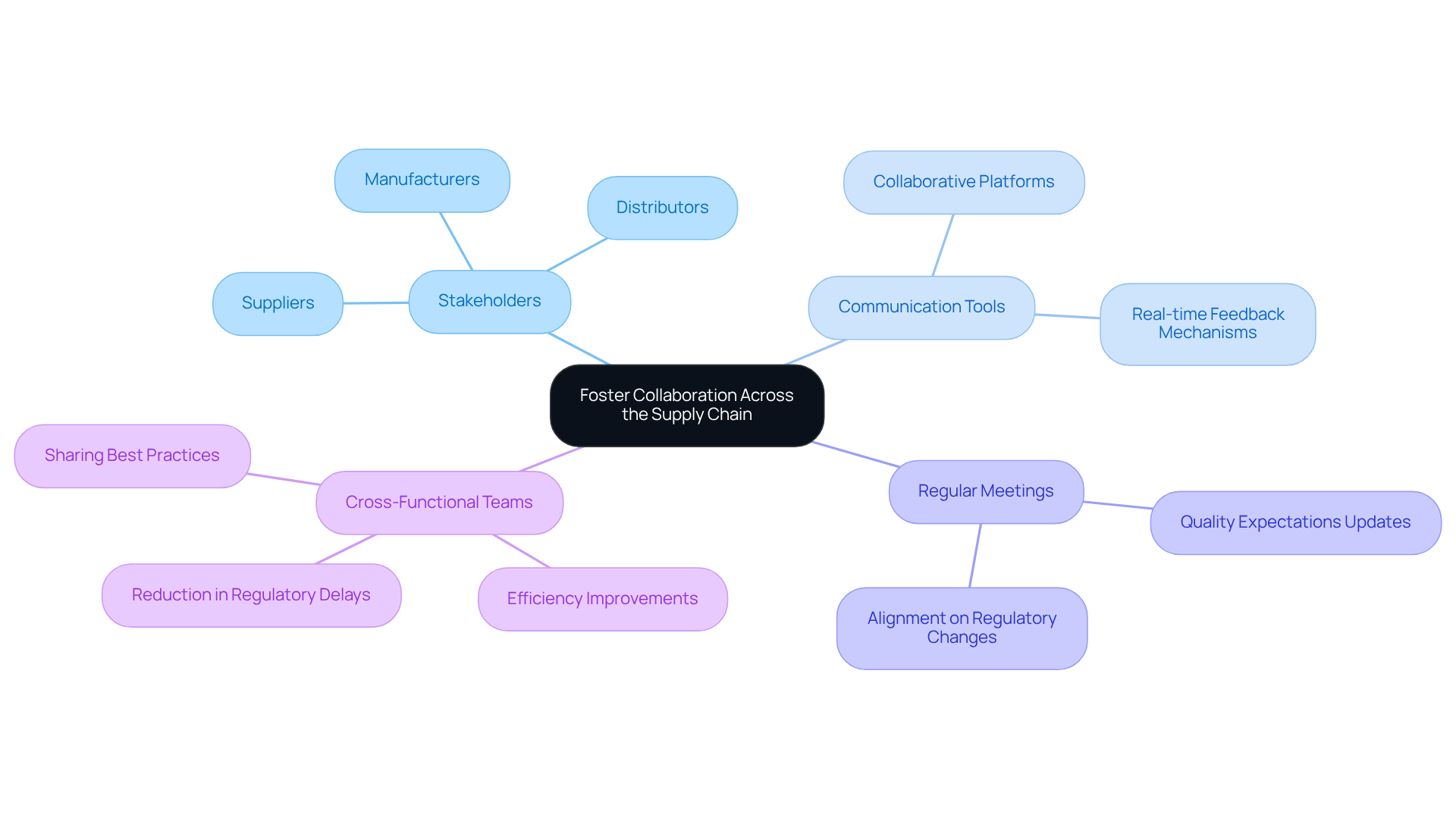
Fostering collaboration across the supply chain is essential for engaging all stakeholders—suppliers, manufacturers, and distributors—in compliance discussions. Regular meetings and updates ensure alignment on regulatory changes and quality expectations. By employing collaborative tools and platforms, companies can improve real-time communication and feedback, allowing for quick modifications to processing methods.
For instance, organizations that have embraced a cross-functional team strategy for regulatory adherence have reported significant enhancements in efficiency and a decrease in delays related to regulations. This collaborative effort enables stakeholders to share insights and best practices, ultimately leading to a more compliant and efficient medical packaging process. Not only does this streamline operations, but it also enhances overall compliance, effectively addressing the complexities of today's regulatory landscape.
Conclusion
Understanding and implementing effective strategies for medical packaging compliance is critical for ensuring product safety and regulatory adherence. This article underscores the necessity of grasping regulatory requirements, employing design strategies, establishing robust quality control measures, and fostering collaboration across the supply chain. Each of these elements plays a vital role in mitigating risks and enhancing the overall integrity of medical packaging.
Key insights discussed include:
- The importance of staying updated on regulations from organizations like the FDA and ISO.
- The need for child-resistant packaging to protect vulnerable populations.
- The implementation of standard operating procedures to uphold quality standards.
- Fostering collaboration among all stakeholders in the supply chain is essential for streamlining processes and maintaining compliance in a complex regulatory environment.
In an industry where safety and compliance are paramount, organizations must prioritize these strategies to not only meet regulatory demands but also protect consumers and enhance their market reputation. By adopting a proactive and collaborative approach, companies can navigate the challenges of medical packaging compliance effectively, ensuring that their products not only meet safety standards but also contribute positively to public health outcomes.
Frequently Asked Questions
What are the key regulatory organizations involved in medical packaging?
The key regulatory organizations involved in medical packaging include the FDA (Food and Drug Administration), EMA (European Medicines Agency), and ISO (International Organization for Standardization).
What are the main areas of focus for regulatory compliance in medical packaging?
The main areas of focus for regulatory compliance in medical packaging include labeling requirements, material safety, and environmental considerations.
Why is it important to regularly review updates from regulatory bodies?
Regularly reviewing updates from regulatory bodies is essential for maintaining compliance and ensuring that medical packaging adheres to the latest guidelines and requirements.
What does the FDA require for medical packaging?
The FDA requires that all medical packaging effectively safeguards products from contamination and degradation throughout their lifecycle.
What recent action did the FDA take to emphasize the importance of regulatory compliance?
In fiscal year 2023, the FDA issued 24 Warning Letters, highlighting the significance of adhering to regulatory guidelines for medical packaging.
What does Jason Haider, CEO of Xenco Medical, say about the FDA's requirements?
Jason Haider states that due to the FDA's strict criteria for the sterility of medical devices, manufacturers must undergo comprehensive testing to demonstrate that medical packaging can withstand environmental influences that could jeopardize the sterility of its contents.
What is the Quality Management System Regulation (QMSR) Final Rule, and when will it take effect?
The Quality Management System Regulation (QMSR) Final Rule emphasizes the necessity for compliance in medical packaging and will take effect on February 2, 2026.
How can manufacturers ensure compliance with medical packaging regulations?
Manufacturers can ensure compliance by engaging with legal professionals or regulatory advisors for insights into specific criteria relevant to their products and by adopting a proactive, technology-driven approach to streamline the validation process and enhance overall efficiency.




