Overview
Achieving packaging compliance for nutraceuticals necessitates a comprehensive understanding and implementation of key regulations concerning labeling, health claims, protection guidelines, and good manufacturing practices. This article delineates specific steps essential for compliance, including:
- The selection of compliant materials
- The design for clarity
- The establishment of robust quality control measures
Collectively, these actions not only ensure adherence to FDA standards but also enhance consumer trust in nutraceutical products.
Introduction
Achieving packaging compliance in the nutraceutical industry transcends mere regulatory obligation; it is a pivotal element that significantly influences consumer trust and brand reputation. As we approach the evolving regulations set to take effect in 2025, it becomes imperative for manufacturers to grasp the intricate requirements—from labeling standards to health claims.
How can companies guarantee that their packaging not only adheres to legal standards but also resonates with health-conscious consumers? This article delineates four essential steps to assist businesses in achieving packaging compliance, safeguarding product integrity, and ultimately bolstering their market position.
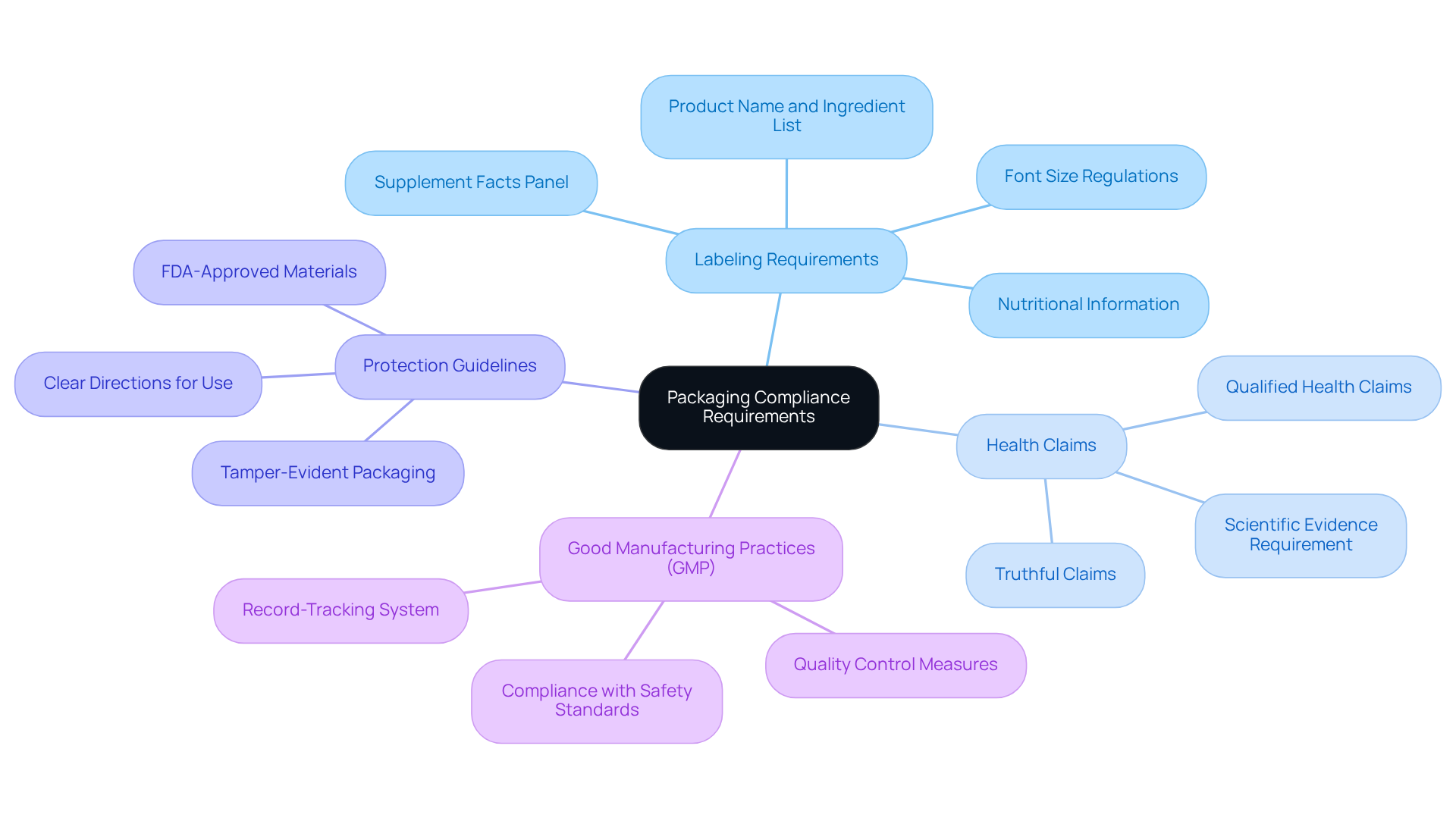
Understand Packaging Compliance Requirements
To achieve packaging compliance for nutraceuticals, it is essential to familiarize yourself with the relevant regulations set forth by the FDA and other regulatory authorities. Focus on the following key areas:
-
Labeling Requirements: Your product labels must include crucial information such as the product name, ingredient list, and nutritional information. It is vital to understand the 'Supplement Facts' panel requirements, which now mandate bolding the type size for key declarations like 'Calories' and 'Serving Size.' Furthermore, the font size for the identity statement must be at least half the size of the largest font on the package, ensuring clarity and compliance.
-
Health Claims: Grasp the guidelines surrounding health assertions on containers. Claims must be truthful, not misleading, and substantiated by scientific evidence. For instance, qualified health claims necessitate a disclaimer indicating that the statement has not been evaluated by the FDA, reinforcing the importance of transparency in marketing.
-
Protection Guidelines: Packaging must adhere to protection guidelines to safeguard the product from contamination and degradation. This includes utilizing FDA-approved materials and ensuring that containers are tamper-evident. Recent updates underscore the necessity of clear directions for use and dosage information, which are vital for consumer safety.
-
Good Manufacturing Practices (GMP): Compliance with GMP guidelines is crucial to maintain item integrity and quality throughout the packaging process. This involves establishing a robust record-tracking system for handling adverse events, which is essential for meeting regulatory requirements.
By thoroughly understanding the regulatory requirements surrounding packaging compliance, you can mitigate potential legal issues and bolster consumer trust in your products. Staying informed about evolving regulations, including recent updates for 2025, will further enhance your capability to navigate the intricate landscape of nutraceutical containers.
Implement Key Compliance Steps in Packaging Design
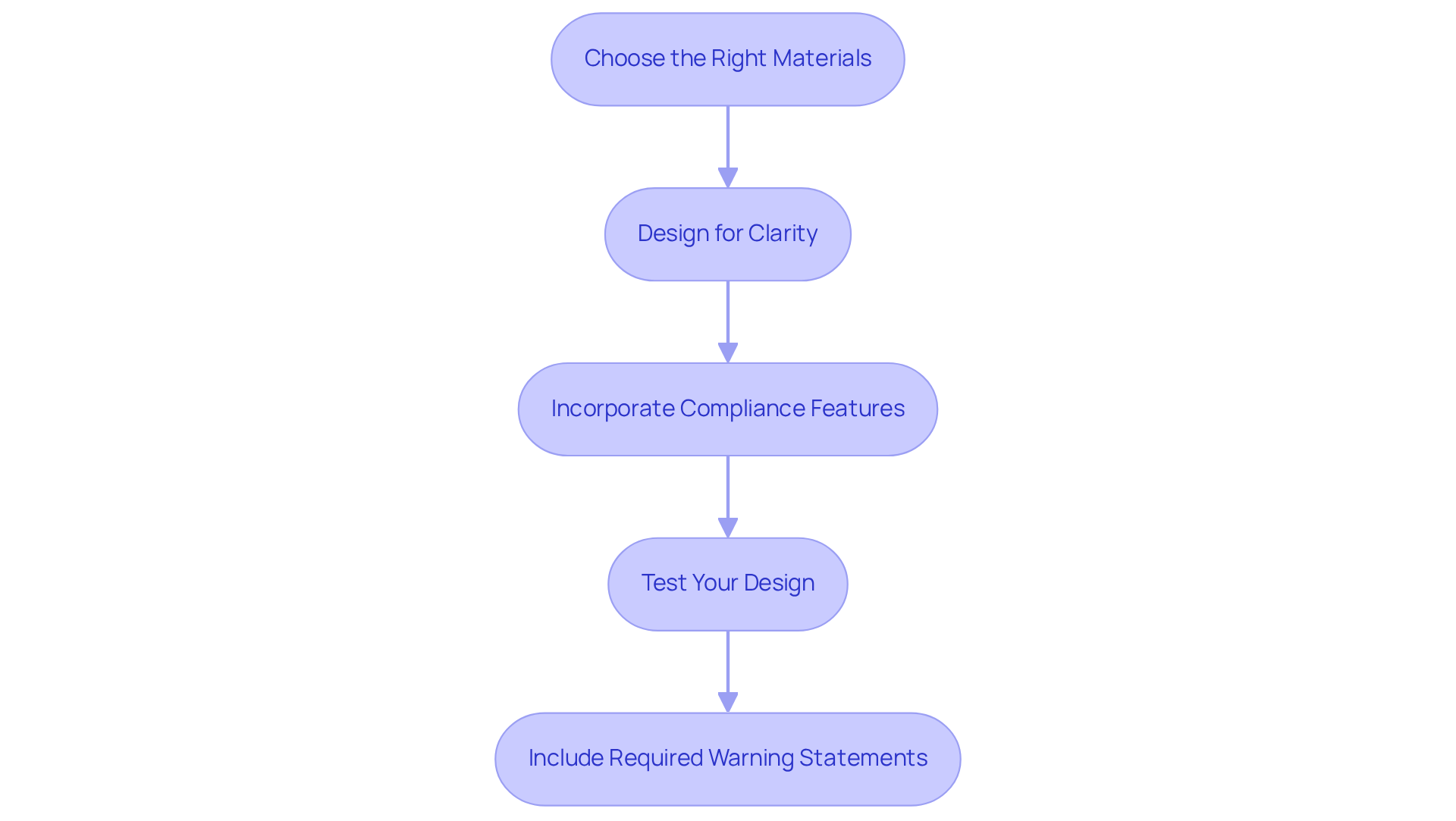
To effectively implement compliance requirements in your packaging design, follow these essential steps:
-
Choose the Right Materials: Opt for packaging materials that comply with FDA regulations, emphasizing moisture resistance, light protection, and child protection. The application of high-barrier films can safeguard supplements from degradation, ensuring product integrity and packaging compliance.
-
Design for Clarity: Make all required information easily visible and legible. Utilize contrasting colors and readable fonts, adhering to FDA guidelines that specify letters must be at least 1/16th inch in height. Clear labeling not only enhances readability but also improves consumer trust, with studies showing that 94% of consumers prefer brands that offer transparency in labeling.
-
Incorporate packaging compliance features by integrating protective elements such as tamper-evident seals and child-resistant closures. These elements not only improve safety but also correspond with consumer preferences, as 68% of consumers prefer tamper-evident wrappers for added security.
-
Test Your Design: Conduct thorough testing of your containers to ensure it meets all regulatory requirements and maintains item integrity under various conditions. This step is vital, as packaging compliance with regulations can lead to a 17% rise in sales and a 28% enhancement in consumer trust.
-
Include Required Warning Statements: For supplements containing iron, ensure that the container includes the FDA-required warning statement: "WARNING: Accidental overdose of iron-containing items is a leading cause of fatal poisoning in children under 6." Keep this item out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately.
By following these steps, you can create containers that ensure packaging compliance with regulatory standards and resonate with consumers, ultimately enhancing your brand's appeal in the competitive nutraceutical market.
Establish Quality Control Measures for Packaging
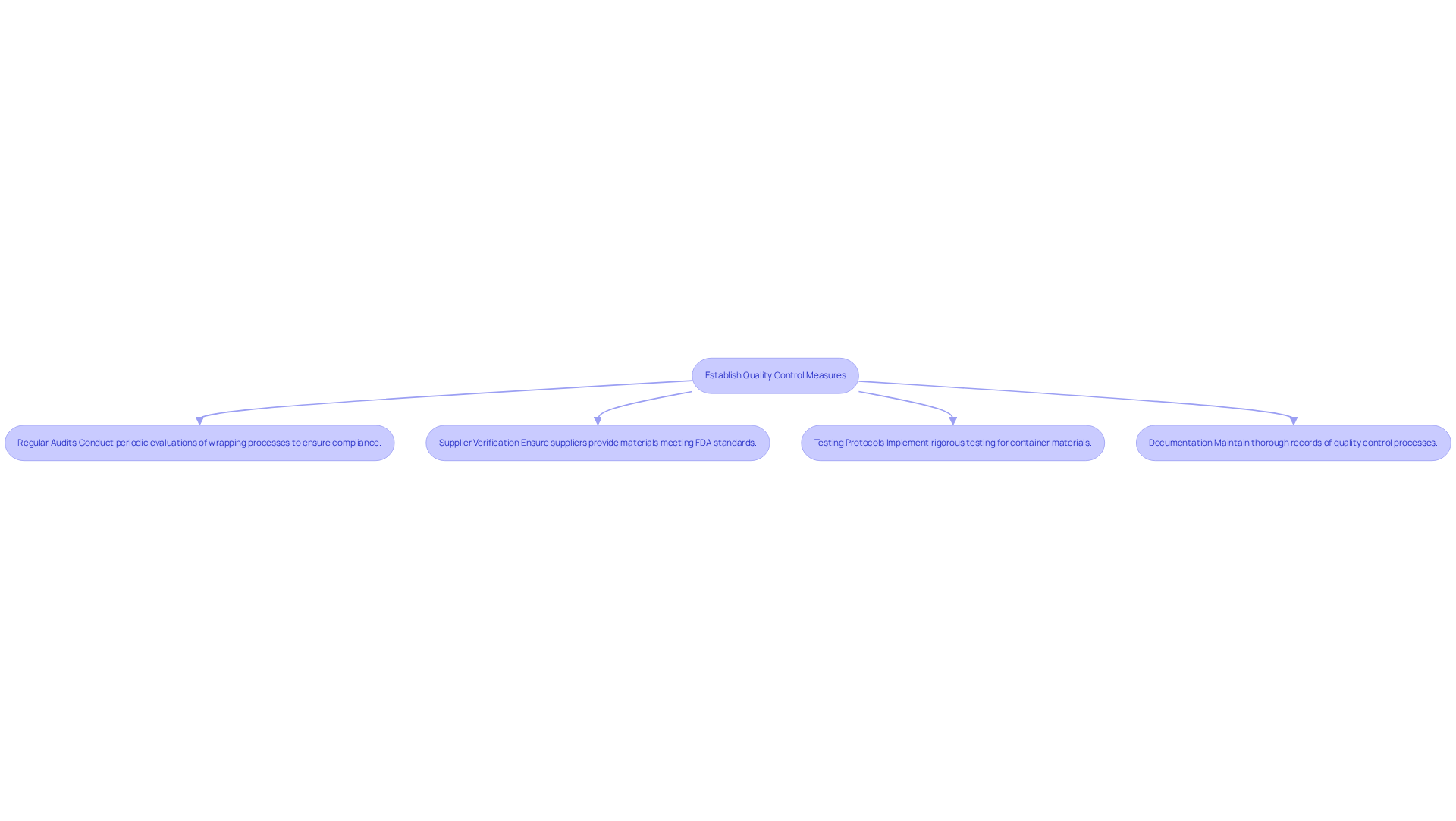
To uphold regulations and guarantee the safety of goods, implementing robust quality control practices for your containers is essential. Here are key practices to consider:
-
Regular audits: Conduct periodic evaluations of your wrapping processes to ensure packaging compliance with the necessary standards. These audits are instrumental in identifying areas for improvement and mitigating risks associated with packaging compliance, which can lead to costly lawsuits and product recalls. For instance, thorough inspections of packaging compliance are recommended several times a year to ensure integrity and quality. Neglecting these inspections can expose your organization to significant risks, including lawsuits and fines.
-
Supplier Verification: Ensure that all suppliers provide materials that meet FDA standards. Request Certificates of Analysis (COA) to verify compliance with safety and quality regulations. This step is crucial; neglecting supplier verification can result in substantial legal repercussions and damage to your brand's reputation, making packaging compliance essential. As highlighted in the industry, effective supplier verification processes are vital for ensuring consistent product quality and preventing disruptions in the nutraceutical supply chain.
-
Testing Protocols: Implement rigorous testing protocols for container materials to assess their integrity and performance. This includes conducting moisture and oxygen barrier tests, dye testing, and bubble leak testing to ensure packaging integrity. Regular testing can help maintain packaging compliance, preventing issues that undermine safety and quality. For instance, performance testing exposes products to real-world conditions, confirming intended performance and ensuring adherence to regulatory standards.
-
Documentation: Maintain thorough records of all quality control processes, including testing results and regulatory checks. This documentation is invaluable during inspections or audits, providing a clear record of adherence to regulatory standards and facilitating smoother packaging compliance verification. Neglecting documentation can lead to significant legal repercussions, underscoring the importance of maintaining accurate records.
By implementing these quality control measures, you can ensure packaging compliance for your containers and effectively safeguard the integrity of your nutraceutical items. Regular inspections and a proactive approach to supplier verification will not only enhance product safety but also bolster consumer trust in your brand.
Optimize Supply Chain for Efficient Packaging Compliance
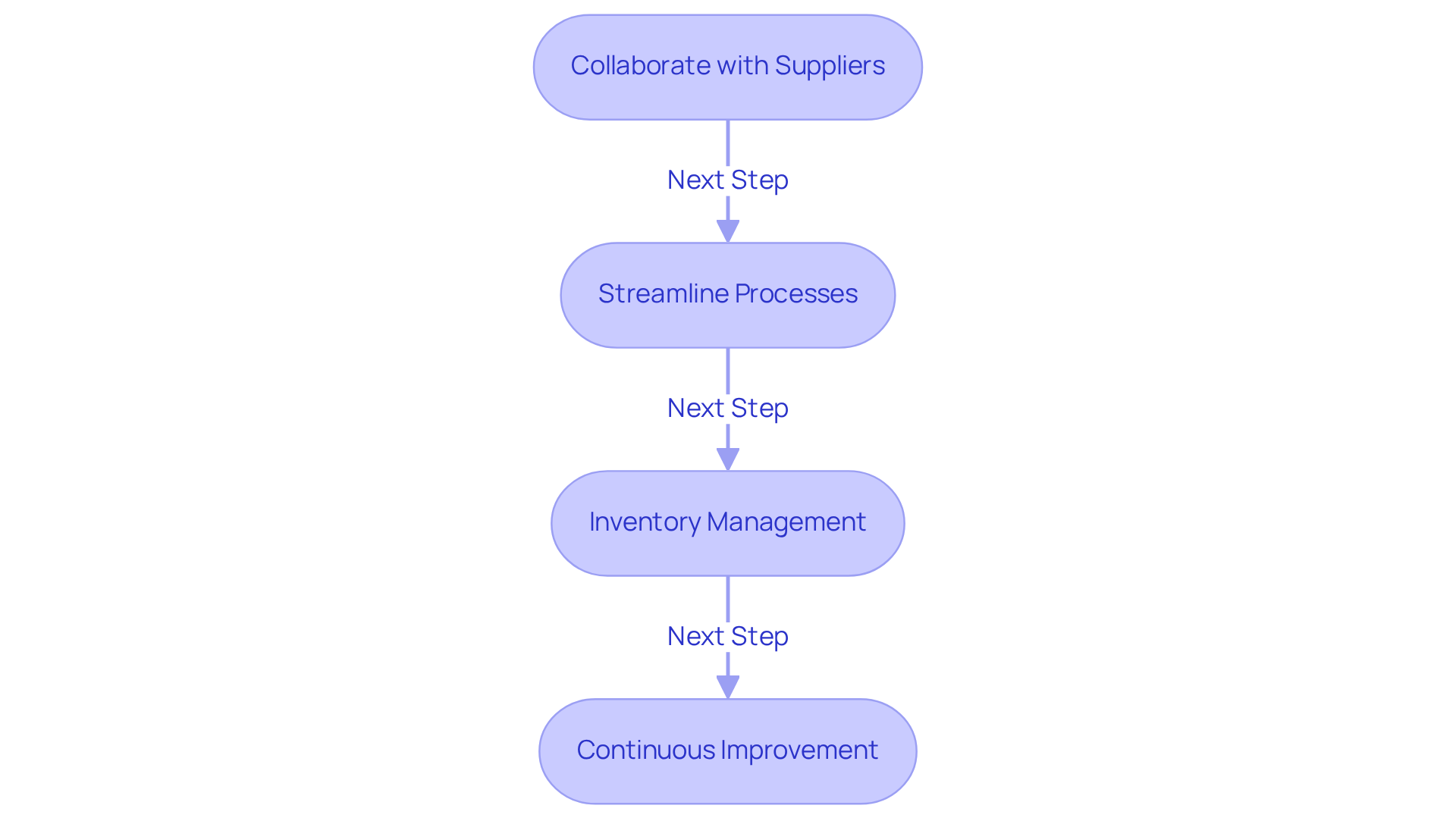
To achieve packaging compliance efficiently, it is essential to optimize your supply chain. Consider these key steps:
-
Collaborate with Suppliers: Engage closely with your suppliers to ensure they fully understand your compliance requirements. This partnership is vital for obtaining materials that ensure packaging compliance with strict regulatory standards, particularly in the nutraceutical industry, where adherence to guidelines is crucial to avoid penalties such as recalls.
-
Streamline Processes: Identify and address bottlenecks in your packing operations. Implementing new technologies or enhancing communication between teams can significantly improve efficiency. For instance, firms that have embraced innovative wrapping technologies have observed enhancements in both compliance and item integrity.
-
Inventory Management: Maintain a well-organized inventory system to effectively track packaging materials. This ensures that compliant materials are readily available when needed, reducing the risk of using non-compliant options that could jeopardize product safety and brand reputation.
-
Continuous Improvement: Regularly review and refine your supply chain processes to adapt to evolving regulations and market demands. Staying informed about industry trends and regulatory updates is crucial for upholding standards. Companies that proactively enhance their supply chain management are better positioned to respond to changes and mitigate risks associated with non-compliance.
By optimizing your supply chain through these strategies, you can enhance operational efficiency and ensure that your packaging compliance consistently meets all necessary standards.
Conclusion
Achieving packaging compliance for nutraceuticals is a multifaceted process that necessitates a thorough understanding of regulations, careful design, and rigorous quality control. By concentrating on the essential requirements established by regulatory authorities such as the FDA, businesses can ensure their products not only meet legal standards but also cultivate consumer trust and safety.
This article outlines critical steps to navigate this complex landscape, including:
- Comprehending labeling requirements
- Implementing good manufacturing practices
- Optimizing supply chains
Key insights underscore the importance of:
- Clear communication on packaging
- Utilizing compliant materials
- Regular audits and testing protocols
Collectively, these strategies contribute to maintaining product integrity and enhancing brand reputation in a competitive market.
Ultimately, the significance of packaging compliance transcends regulatory adherence; it shapes consumer perceptions and influences purchasing decisions. As the nutraceutical industry evolves, staying informed about upcoming regulations and continually refining packaging practices will be essential. Embracing these best practices not only safeguards businesses against potential legal issues but also positions them as leaders in transparency and quality within the nutraceutical sector.
Frequently Asked Questions
What are the key areas to focus on for packaging compliance in nutraceuticals?
The key areas include labeling requirements, health claims, protection guidelines, and Good Manufacturing Practices (GMP).
What must be included on product labels for nutraceuticals?
Product labels must include the product name, ingredient list, nutritional information, and comply with 'Supplement Facts' panel requirements, including specific font size regulations.
What are the requirements for the 'Supplement Facts' panel?
The 'Supplement Facts' panel must have key declarations like 'Calories' and 'Serving Size' in bold type size, and the identity statement font must be at least half the size of the largest font on the package.
What guidelines exist for health claims on nutraceutical packaging?
Health claims must be truthful, not misleading, and substantiated by scientific evidence. Qualified health claims require a disclaimer stating that the statement has not been evaluated by the FDA.
What are the protection guidelines for nutraceutical packaging?
Packaging must use FDA-approved materials, be tamper-evident, and include clear directions for use and dosage information to ensure consumer safety.
Why are Good Manufacturing Practices (GMP) important for packaging compliance?
GMP guidelines help maintain product integrity and quality during the packaging process and require a robust record-tracking system for managing adverse events.
How can staying informed about packaging regulations benefit nutraceutical businesses?
Staying informed about evolving regulations can help mitigate potential legal issues and enhance consumer trust in products, especially with updates anticipated for 2025.




