Overview
The article delineates four pivotal strategies for effective nutritional supplement packaging. It emphasizes the critical nature of:
- Regulatory compliance
- Quality control
- Innovative design
- Integrated filling services
Each strategy is substantiated by compelling evidence, such as the necessity of adhering to FDA guidelines to foster consumer trust. Moreover, it highlights the significant impact of appealing packaging on purchasing decisions and the operational efficiencies achieved through integrated services. These strategies collectively aim to enhance product integrity and bolster market competitiveness.
Introduction
Navigating the intricate world of nutritional supplement packaging presents a unique set of challenges and opportunities for manufacturers. With regulatory compliance at the forefront, companies must not only adhere to strict guidelines but also leverage innovative strategies to enhance market appeal and operational efficiency. As competition intensifies, the pressing question emerges: how can producers effectively balance compliance, quality, and creativity in their packaging to distinguish themselves in a crowded marketplace? This article explores four essential strategies that can elevate packaging from a mere necessity into a powerful tool for brand success.
Understand Regulatory Compliance in Nutritional Supplement Packaging
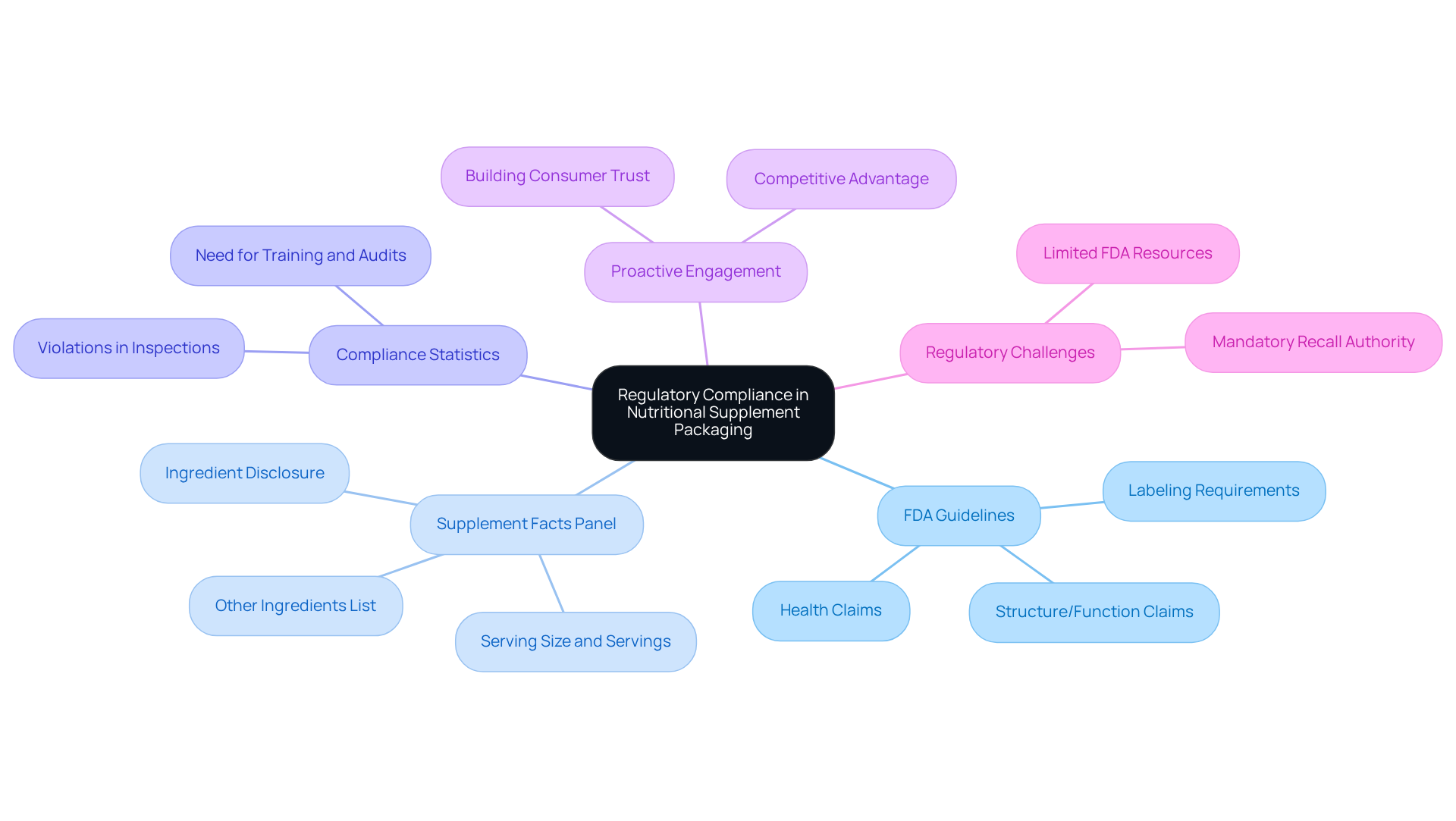
Producers must familiarize themselves with relevant regulations when navigating the complex landscape of nutritional supplement packaging. Understanding the FDA's guidelines on labeling is essential, as these regulations mandate clear and accurate information regarding ingredients, serving sizes, and health claims. Notably, the 'Supplement Facts' panel must be prominently displayed and adhere to specific formatting rules.
Furthermore, producers must stay informed about any changes in regulations, such as the FDA's proposed front-of-package nutrition labeling, aimed at enhancing public awareness. Compliance statistics reveal that over half of inspected dietary supplement producers have violations, underscoring the necessity of regular training and audits to ensure adherence and mitigate risks associated with non-compliance, including product recalls or legal penalties.
Industry leaders assert that proactive engagement with regulatory requirements not only builds consumer trust but also positions companies advantageously in a competitive market. Additionally, the AMA endorses mandatory recall authority over dietary supplements containing drugs or drug analogues, highlighting the regulatory challenges faced by producers. Given the FDA's limited resources for analyzing dietary supplements, it is imperative for producers to remain vigilant in their compliance efforts.
Implement Quality Control Measures for Packaging Integrity
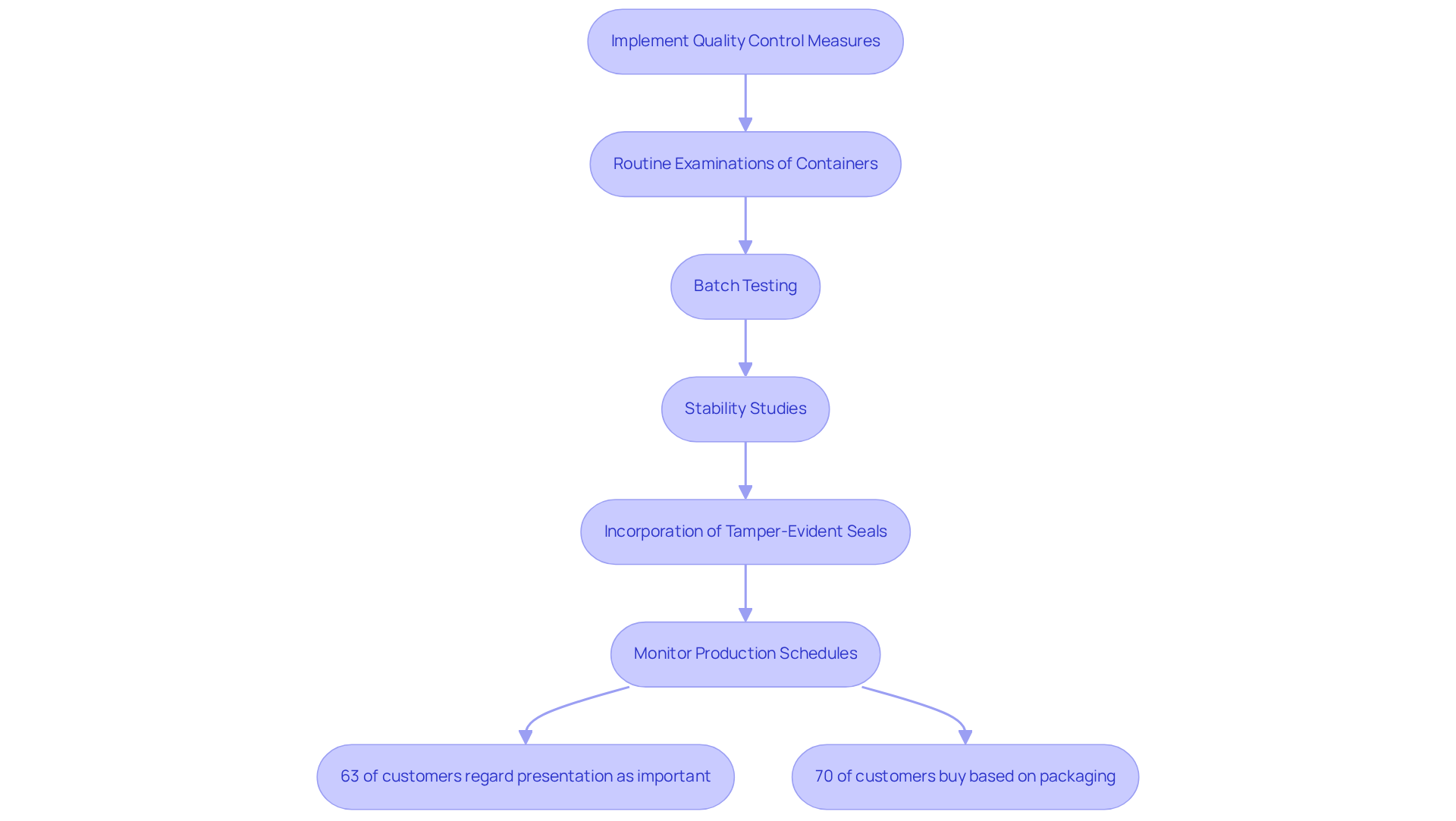
To safeguard the integrity of nutritional supplements, manufacturers must implement stringent quality control measures throughout the wrapping process. Routine examinations of containers are essential to ensure compliance with safety regulations and to eliminate contaminants. Techniques such as batch testing and stability studies are crucial for confirming that products maintain their potency and quality over time. The incorporation of tamper-evident seals and child-resistant containers not only enhances safety for users but also cultivates trust in the product.
These seals serve as a critical barrier against tampering, ensuring that consumers receive products in their intended condition, which is increasingly significant as:
- 63% of customers regard presentation as equally important as the label itself.
Moreover, inadequate wrapping can lead to substantial financial losses and damage to a company's reputation, making it imperative for producers to oversee quality checkpoints along the packing line. By fostering a culture of quality within the organization and leveraging automation and data analytics to monitor production schedules and defect rates, manufacturers can significantly mitigate the risk of quality-related issues, ultimately bolstering their brand reputation.
This proactive approach is vital, as:
- 70% of customers admit to purchasing products based solely on the appeal of the design.
Leverage Innovative Packaging Design to Enhance Market Appeal
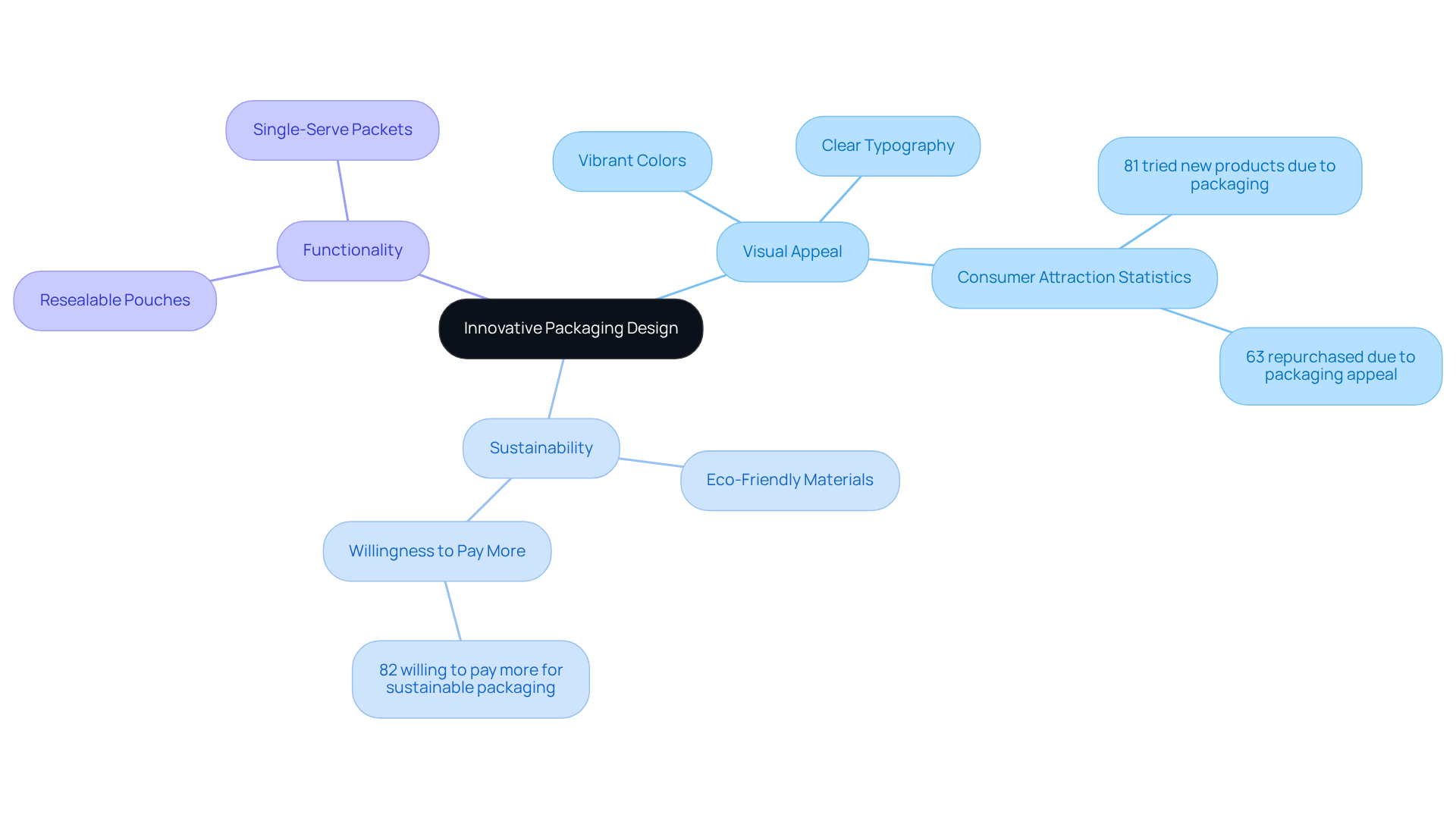
In the competitive environment of nutritional supplements, manufacturers must emphasize creative nutritional supplement packaging that appeals to buyers. Employing vibrant hues and simple designs can significantly enhance product visibility; research indicates that 81% of shoppers are drawn to items based on their presentation. Clear typography further aids in effectively conveying essential information.
Additionally, the incorporation of sustainable materials in nutritional supplement packaging is crucial, as 70% of individuals consider themselves environmentally conscious, with 82% willing to spend more for eco-friendly options. Functional features, such as resealable pouches and single-serve packets in nutritional supplement packaging, enhance convenience and usability, aligning with user expectations for practicality.
Furthermore, companies should recognize that 52% of customers have switched labels due to new designs, underscoring the importance of maintaining attractive visuals. By strategically aligning product design with these preferences and current market trends, brands can elevate their visibility, foster customer loyalty, and drive sales.
Integrate Filling Services with Packaging Solutions for Operational Efficiency
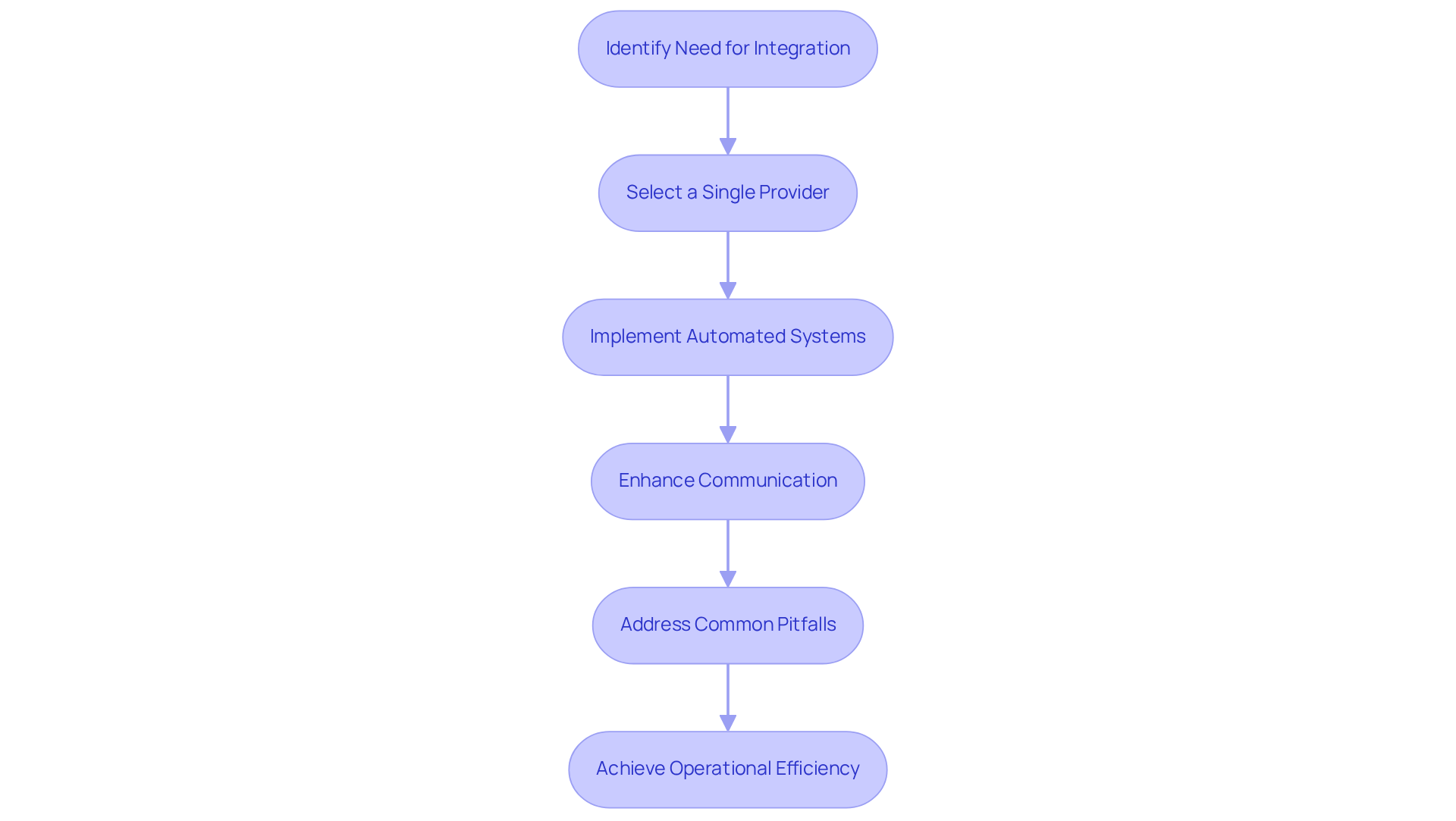
To achieve operational effectiveness, producers must consider integrating their filling services with wrapping solutions. This can be realized by partnering with a single provider that delivers both services, which ensures seamless communication and coordination. For example, employing automated filling lines directly connected to wrapping machines can significantly reduce handling times and mitigate the risk of contamination.
Furthermore, this strategy enables real-time adjustments based on production demands, enhancing responsiveness and flexibility. By streamlining these processes, manufacturers can boost productivity and lower costs, ultimately benefiting their bottom line. Notably, statistics indicate that material inefficiencies contribute to as much as 30% of production downtime within the industry, underscoring the importance of integration.
As Asif Muhammad, Co-Founder and Partner at Refine Packaging, aptly states, "A dedicated specialist will guide you through every step of the custom wrapping process without breaking a sweat." This statement reinforces the value of having a single provider for both filling and wrapping services.
Additionally, a case study focusing on improving packaging efficiency for CPG brands demonstrates how integrated approaches can transform packaging from a cost center into a high-performance asset. However, producers must remain vigilant regarding common pitfalls, such as miscommunication between teams or insufficient training on new systems, which can impede the effectiveness of integration.
By leveraging the capabilities of a single provider, manufacturers can enhance their overall operational agility and market competitiveness.
Conclusion
Navigating the complexities of nutritional supplement packaging necessitates a multifaceted approach that emphasizes regulatory compliance, quality control, innovative design, and operational efficiency. By comprehensively understanding and adhering to FDA guidelines, producers can cultivate consumer trust and protect their market position. This foundational knowledge not only mitigates risks associated with non-compliance but also promotes a culture of accountability within organizations.
Key strategies include:
- Implementing rigorous quality control measures to ensure packaging integrity.
- Leveraging attractive and sustainable design elements that resonate with consumer preferences.
- Integrating filling services with packaging solutions to enhance operational efficiency.
These practices are essential in a competitive landscape where presentation and usability significantly influence consumer purchasing decisions. Notably, statistics indicate that a considerable percentage of customers are inclined to switch brands based solely on packaging, highlighting the critical importance of thoughtful design and execution.
In conclusion, the significance of effective nutritional supplement packaging cannot be overstated. As the industry evolves, producers must remain vigilant and adaptable, embracing best practices that not only meet regulatory demands but also align with consumer expectations. By investing in innovative strategies and fostering a commitment to quality, manufacturers can enhance their product offerings, drive customer loyalty, and achieve long-term success in the marketplace.
Frequently Asked Questions
What regulations must producers understand for nutritional supplement packaging?
Producers must familiarize themselves with the FDA's guidelines on labeling, which require clear and accurate information regarding ingredients, serving sizes, and health claims.
What is the importance of the 'Supplement Facts' panel?
The 'Supplement Facts' panel must be prominently displayed on packaging and adhere to specific formatting rules as mandated by regulations.
Why is it necessary for producers to stay informed about regulatory changes?
Staying informed about changes, such as the FDA's proposed front-of-package nutrition labeling, is essential for enhancing public awareness and ensuring compliance.
What do compliance statistics reveal about dietary supplement producers?
Compliance statistics indicate that over half of inspected dietary supplement producers have violations, highlighting the need for regular training and audits.
What are the risks associated with non-compliance in nutritional supplement packaging?
Non-compliance can lead to product recalls or legal penalties, making adherence to regulations crucial for producers.
How does proactive engagement with regulatory requirements benefit companies?
Proactive engagement builds consumer trust and positions companies advantageously in a competitive market.
What stance does the AMA take regarding dietary supplements containing drugs?
The AMA endorses mandatory recall authority over dietary supplements that contain drugs or drug analogues due to regulatory challenges.
Why is it crucial for producers to remain vigilant in their compliance efforts?
Given the FDA's limited resources for analyzing dietary supplements, producers must remain vigilant to ensure compliance and mitigate risks.




