Overview
The article delineates five essential steps to master aseptic manufacturing for nutraceuticals, emphasizing the principles of:
- Sterilization
- Facility preparation
- Staff training
- Quality control
- Integration into production processes
Each step underscores the critical importance of maintaining a sterile environment and adhering to rigorous protocols to ensure product safety and quality. This meticulous approach not only fosters consumer trust but also ensures compliance with safety standards, reinforcing the necessity for quality solutions in the industry.
Introduction
Aseptic manufacturing stands as a cornerstone of nutraceutical production, effectively safeguarding products from contamination and ensuring consumer safety. By mastering the principles of this meticulous process, manufacturers not only enhance product quality but also foster consumer trust in their brands.
However, the journey toward excellence in aseptic manufacturing presents significant challenges—how can companies adeptly implement these complex protocols while sustaining efficiency and compliance? This inquiry invites a deeper exploration into the strategies that can empower manufacturers to navigate these hurdles successfully.
Understand Aseptic Manufacturing Principles
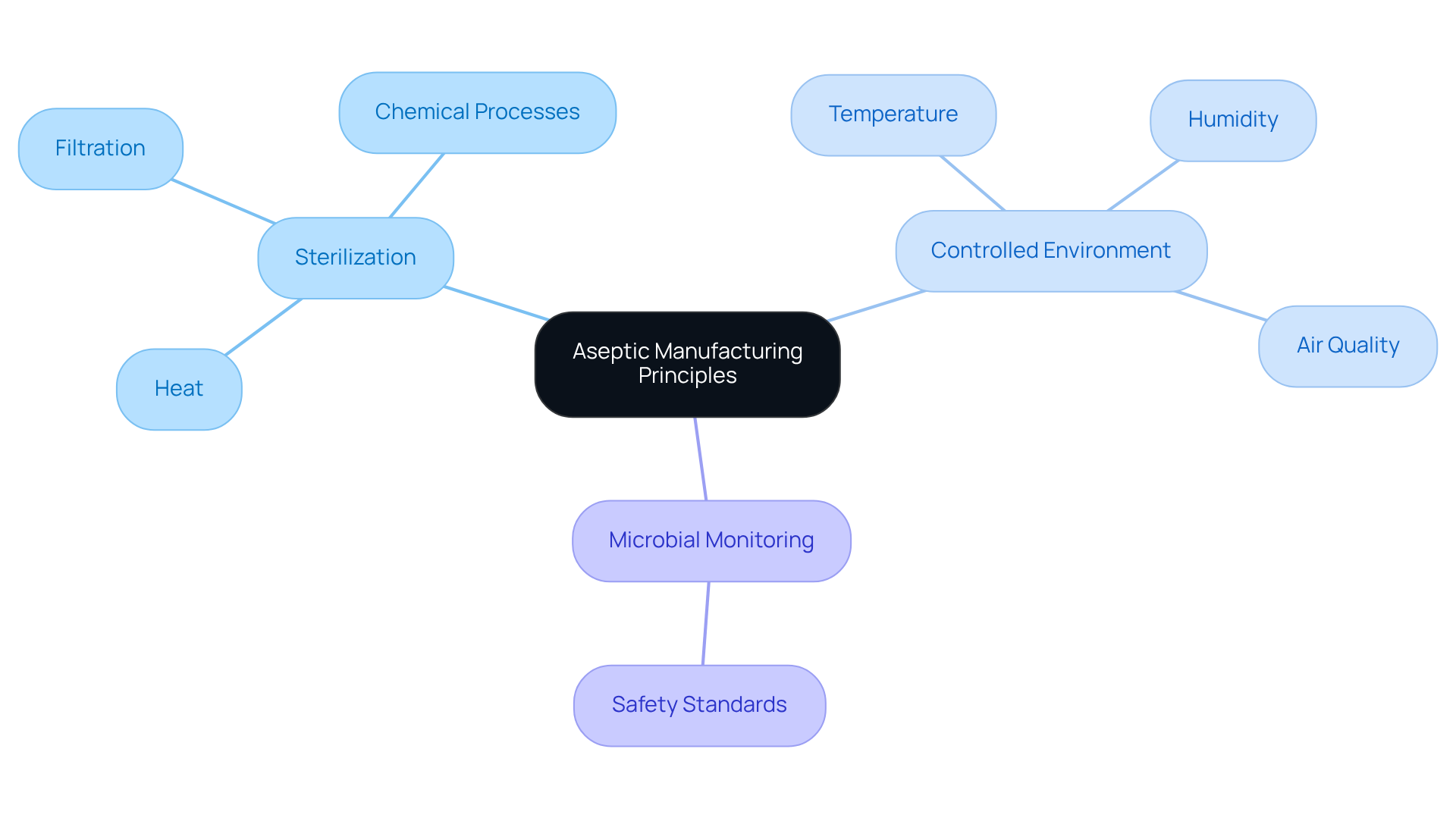
Aseptic manufacturing is a critical method that ensures items remain free from contamination by pathogens and spoilage organisms. This process necessitates the separate sterilization of both the product and its packaging before their combination in a sterile environment. The key principles of aseptic manufacturing include:
- Sterilization: Grasp the various methods of sterilization—such as heat, filtration, and chemical processes—and their applicability to different types of nutraceuticals.
- Controlled Environment: Recognize the paramount importance of maintaining a controlled environment for aseptic manufacturing, which includes regulating temperature, humidity, and air quality to effectively prevent contamination.
- Microbial Monitoring: Understand the significance of monitoring microbial levels within both the facility and the products to ensure adherence to safety standards in aseptic manufacturing.
By mastering these principles, you lay the groundwork for efficient sterile manufacturing in your nutraceutical production line, establishing a reliable foundation for quality and safety.
Prepare Your Facility and Equipment
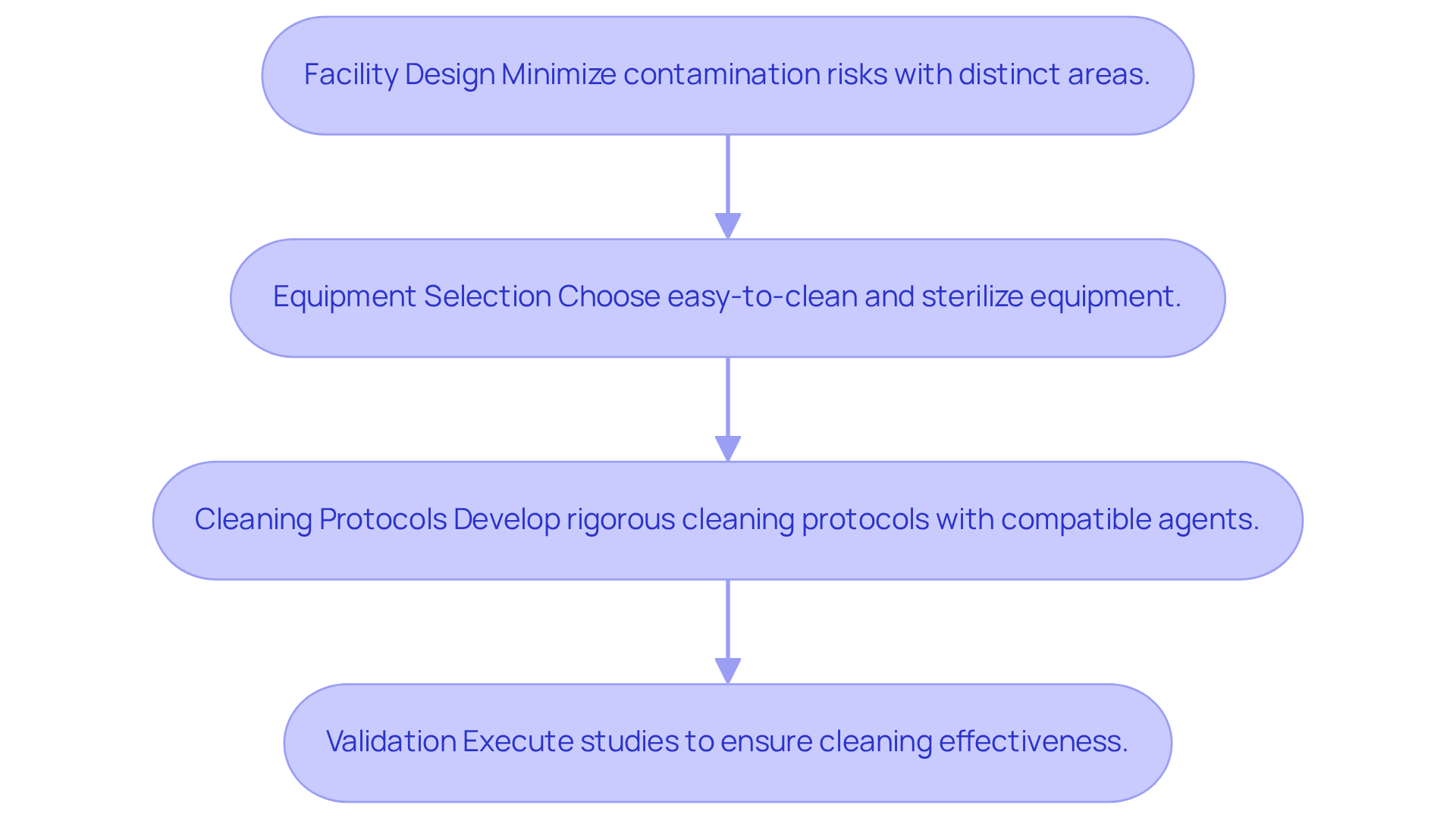
To prepare your facility and equipment for aseptic manufacturing, follow these essential steps:
- Facility Design: Ensure that your facility is meticulously designed to minimize contamination risks. This includes establishing distinct areas for raw materials, production, and packaging.
- Equipment Selection: Opt for equipment that is straightforward to clean and sterilize. Consider implementing automated systems that significantly reduce human intervention.
- Cleaning Protocols: Develop rigorous cleaning protocols for all surfaces and equipment. Utilize appropriate disinfectants and confirm that all cleaning agents are compatible with your products.
- Validation: Execute validation studies to ensure that your cleaning and sterilization processes are effective. This may involve testing for residual contaminants after cleaning.
By diligently preparing your facility and equipment, you lay a robust foundation for successful aseptic manufacturing.
Train Staff on Aseptic Techniques
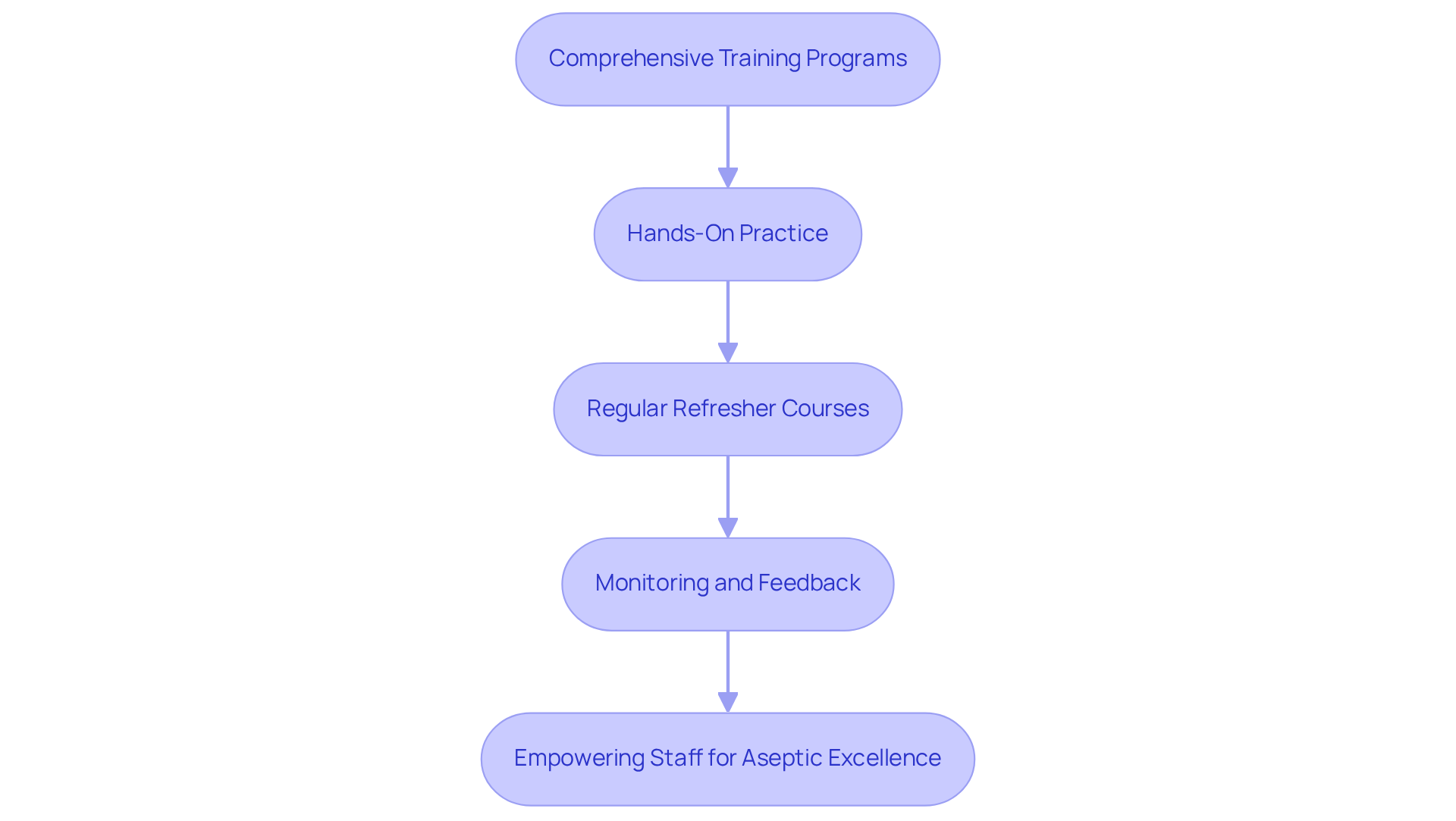
Training your staff on aseptic techniques is essential for maintaining the highest standards in aseptic manufacturing.
-
Comprehensive Training Programs: Develop training programs that cover the principles of sterile manufacturing, emphasizing the importance of personal hygiene and proper gowning procedures.
-
Hands-On Practice: Provide practical training sessions where staff can apply sterile techniques in a controlled environment, reinforcing their learning through real-world application.
-
Regular Refresher Courses: Schedule regular refresher courses to keep staff informed about best practices and any updates in protocols, ensuring continuous improvement.
-
Monitoring and Feedback: Implement a robust system for observing staff compliance with sterile techniques, offering constructive feedback to reinforce positive behaviors.
By investing in comprehensive staff training, you empower your team to uphold the highest standards of aseptic manufacturing, ensuring reliability and excellence in your operations.
Implement Quality Control Protocols
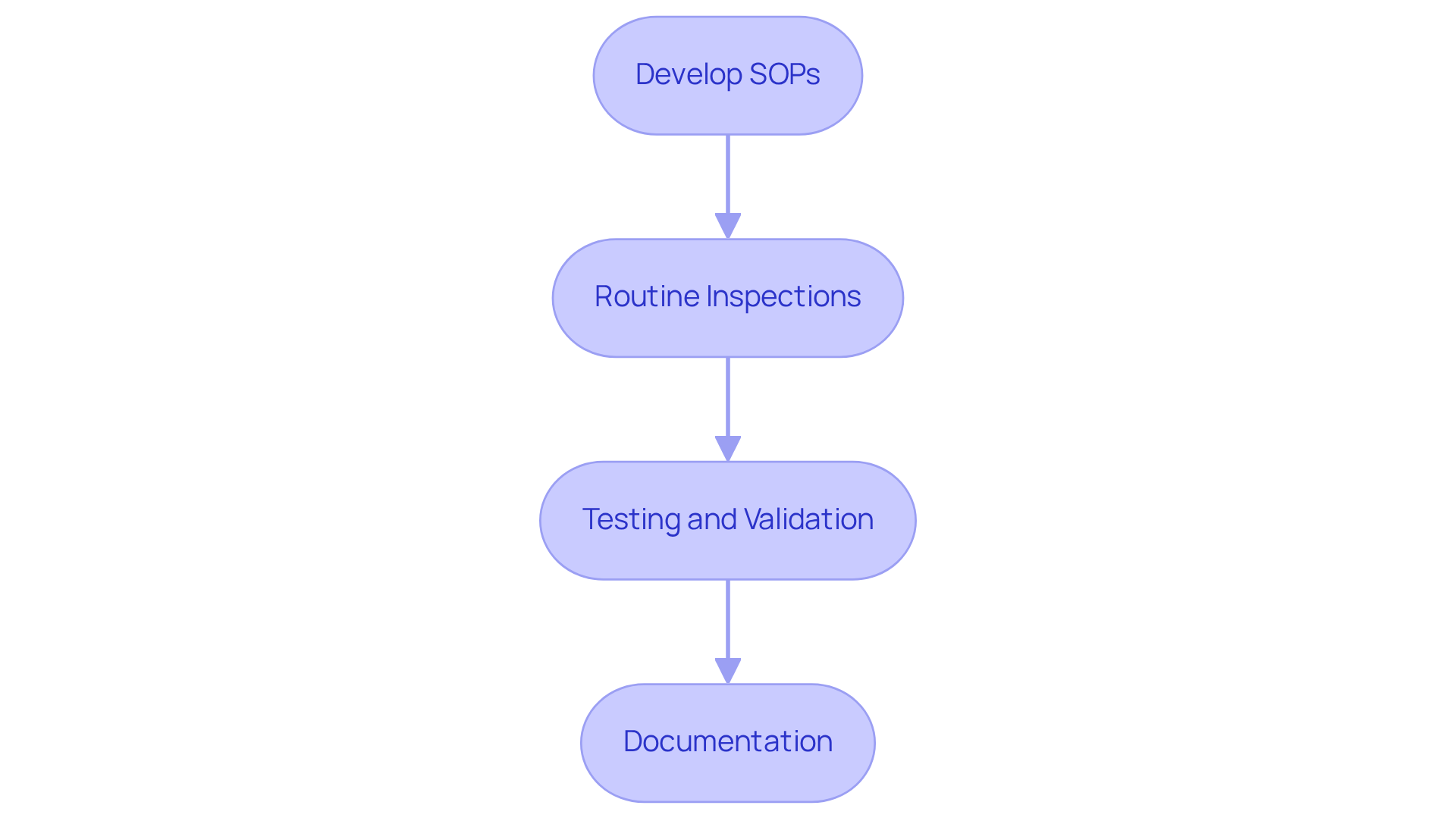
To implement effective quality control protocols, consider the following strategies:
- Develop comprehensive Standard Operating Procedures (SOPs) for every aspect of aseptic manufacturing, encompassing everything from raw material management to final item packaging.
- Routine Inspections: Conduct routine inspections of your procedures and facilities to ensure strict adherence to established protocols.
- Testing and Validation: Regularly test products for microbial contamination and consistently validate your sterilization methods.
- Documentation: Maintain meticulous records of all control measures, including test results and any corrective actions taken.
By establishing robust quality control protocols, you can ensure that your aseptic manufacturing process consistently produces safe and high-quality nutraceuticals.
Integrate Aseptic Processes into Production
To effectively integrate aseptic processes into your production, adhere to the following steps:
- Workflow Design: Establish a production workflow that incorporates sterile techniques at every stage, from raw material handling to final packaging.
- Cross-Functional Collaboration: Foster teamwork among divisions, including production, quality control, and logistics, to ensure a seamless integration of sterile methods.
- Ongoing Enhancement: Cultivate an environment of continuous improvement where feedback from personnel and control results are leveraged to refine sterile procedures.
- Stakeholder Communication: Communicate the importance of sterile procedures to all stakeholders, including suppliers and customers, to ensure alignment and support.
By successfully integrating aseptic processes into your production, you enhance product safety and quality, ultimately fostering greater consumer trust and brand loyalty.
Conclusion
Mastering aseptic manufacturing is essential for ensuring the safety and quality of nutraceutical products. By grasping the principles of sterile production, preparing facilities and equipment, training staff, implementing quality control protocols, and integrating aseptic processes into production, manufacturers can significantly enhance their operational efficiency and product integrity.
Key insights from this guide highlight the necessity of:
- A controlled environment
- Rigorous cleaning protocols
- Comprehensive staff training
Each step in the aseptic manufacturing process, from sterilization techniques to the validation of cleaning methods, is crucial in preventing contamination and ensuring that nutraceuticals meet high safety standards.
Ultimately, the commitment to mastering aseptic manufacturing not only protects consumer health but also cultivates trust and loyalty within the market. By adopting best practices and continuously improving processes, manufacturers can stay ahead of industry standards and deliver high-quality nutraceuticals that consumers can depend on. Embracing these principles is vital for anyone aiming to excel in the nutraceutical sector and contribute to a safer, healthier future.
Frequently Asked Questions
What is aseptic manufacturing?
Aseptic manufacturing is a critical method that ensures items remain free from contamination by pathogens and spoilage organisms. It involves the separate sterilization of both the product and its packaging before their combination in a sterile environment.
What are the key principles of aseptic manufacturing?
The key principles of aseptic manufacturing include sterilization, controlled environment, and microbial monitoring. Sterilization involves various methods like heat, filtration, and chemical processes. A controlled environment maintains regulated temperature, humidity, and air quality, while microbial monitoring ensures adherence to safety standards.
Why is sterilization important in aseptic manufacturing?
Sterilization is essential in aseptic manufacturing to eliminate pathogens and spoilage organisms, ensuring that both the product and its packaging are free from contamination before they are combined.
How does a controlled environment contribute to aseptic manufacturing?
A controlled environment is crucial as it regulates temperature, humidity, and air quality to effectively prevent contamination during the manufacturing process.
What role does microbial monitoring play in aseptic manufacturing?
Microbial monitoring is significant for ensuring that microbial levels within the facility and products adhere to safety standards, thereby maintaining the integrity of the aseptic manufacturing process.
What steps should be taken to prepare a facility for aseptic manufacturing?
To prepare a facility for aseptic manufacturing, ensure meticulous facility design to minimize contamination risks, select equipment that is easy to clean and sterilize, develop rigorous cleaning protocols, and execute validation studies to confirm the effectiveness of cleaning and sterilization processes.
Why is facility design important in aseptic manufacturing?
Facility design is important because it helps minimize contamination risks by establishing distinct areas for raw materials, production, and packaging, thereby maintaining a sterile environment.
What should be considered when selecting equipment for aseptic manufacturing?
Equipment should be straightforward to clean and sterilize, and it may be beneficial to implement automated systems to reduce human intervention, which can introduce contamination risks.
What are cleaning protocols, and why are they necessary?
Cleaning protocols are rigorous procedures developed for all surfaces and equipment, utilizing appropriate disinfectants. They are necessary to ensure that all equipment and surfaces are free from contaminants that could compromise the aseptic manufacturing process.
What does validation involve in the context of aseptic manufacturing?
Validation involves executing studies to ensure that cleaning and sterilization processes are effective, which may include testing for residual contaminants after cleaning.




