Overview
The article delineates seven pivotal benefits of collaborating with contract manufacturing organizations (CMOs) within the pharmaceutical sector. These advantages encompass:
- Cost-effectiveness
- Flexibility
- Access to advanced technologies
- Enhanced quality assurance
Evidence substantiates these benefits, illustrating how CMOs streamline processes, diminish operational costs, and stimulate innovation. This collaboration enables pharmaceutical firms to concentrate on their core competencies while adhering to regulatory standards. By leveraging the expertise of CMOs, companies can ensure reliable and efficient operations, ultimately fostering growth and compliance in a competitive landscape.
Introduction
The pharmaceutical industry is experiencing a transformative shift, propelled by the increasing complexity of regulations and the pressing need for efficiency in production. Contract manufacturing organizations (CMOs) are emerging as pivotal players in this landscape, providing a range of benefits that can significantly enhance operational effectiveness.
As companies confront rising costs and the demand for rapid innovation, a critical question arises: how can partnering with a CMO not only alleviate these pressures but also drive a pharmaceutical firm toward greater success?
By exploring the advantages of CMOs, we uncover a strategic pathway for companies eager to thrive in an ever-evolving market.
Western Packaging: Integrated Packaging Solutions for Pharmaceutical Efficiency
Western Packaging offers a comprehensive suite of services designed to streamline the pharmaceutical packaging process. By integrating cutting-edge packaging design, customized filling services, and extensive third-party logistics (3PL), they create a seamless workflow that reduces lead times and enhances delivery efficiency. Their end-to-end solutions, which include warehousing and inventory management, simplify the supply chain, allowing companies to concentrate on their core competencies. This thorough approach guarantees that products are packaged effectively and attractively, boosting brand visibility and shelf appeal. Trust Western Packaging to elevate your packaging strategy and ensure reliability in every step of the process.
Regulatory Compliance Assurance: Navigating Complex Pharmaceutical Standards
Contract manufacturing organizations pharmaceutical, like Western Packaging, are essential in navigating the intricate regulatory landscape of the pharmaceutical industry. Their profound understanding of FDA regulations and compliance standards guarantees that all packaging and manufacturing processes adhere to stringent requirements. This expertise not only shields companies from potential legal issues but also fosters consumer confidence in their products.
By partnering with contract manufacturing organizations pharmaceutical, businesses can effectively tackle compliance challenges, allowing them to concentrate on product development and marketing strategies. Moreover, CMOs implement rigorous quality control measures and maintain transparent communication with regulatory bodies, crucial for adapting to changing standards. This collaboration not only streamlines operations but also enhances the overall integrity of medical offerings in a competitive market.
Cost-Effectiveness: Reducing Manufacturing Expenses Through Outsourcing
Outsourcing manufacturing to contract manufacturing organizations pharmaceutical can lead to substantial reductions in operational costs for pharmaceutical companies. By leveraging the economies of scale that marketing organizations provide, businesses can significantly reduce their production costs while upholding high-quality standards.
Chief Marketing Officers typically have established supply chains and strong relationships with suppliers, which further contribute to cost reductions. This financial flexibility allows organizations to reallocate resources more efficiently, ultimately enhancing their overall profitability.
According to industry experts, organizations that involve Chief Marketing Officers can save as much as 30% on production expenses, enabling them to allocate more funds to research and development or promotional efforts.
Moreover, the ability to rapidly increase production in response to market needs without the burden of fixed expenses is a crucial benefit that contract manufacturing organizations pharmaceutical provide, establishing them as vital allies in the healthcare industry.
Flexibility and Scalability: Adapting to Market Changes with CDMOs
Contract manufacturing organizations in the pharmaceutical sector are pivotal in enabling pharmaceutical firms to adeptly navigate the complexities of market fluctuations. Their inherent flexibility and scalability facilitate rapid adjustments in production levels, whether responding to the surge of a new product launch or scaling back during periods of reduced demand.
For instance, Curia, a global CDMO with over 30 years of experience, exemplifies this adaptability by providing comprehensive capabilities in Generic APIs while emphasizing open communication and collaboration with clients. This adaptability is essential for optimizing resource allocation and minimizing waste, ensuring that companies remain agile and competitive.
Marketing executives, such as those at Curia, can swiftly increase output to meet unexpected consumer demand, thereby enhancing market responsiveness. Furthermore, their capacity to optimize processes assists drug companies in sustaining efficiency, ultimately resulting in enhanced profitability and sustainability within a dynamic industry environment.
With a network of 23 integrated facilities and a team of highly-trained professionals, Curia specializes in clinical and commercial sterile manufacturing for Biologics, demonstrating the scale and capability of contract manufacturers in adapting to market changes.
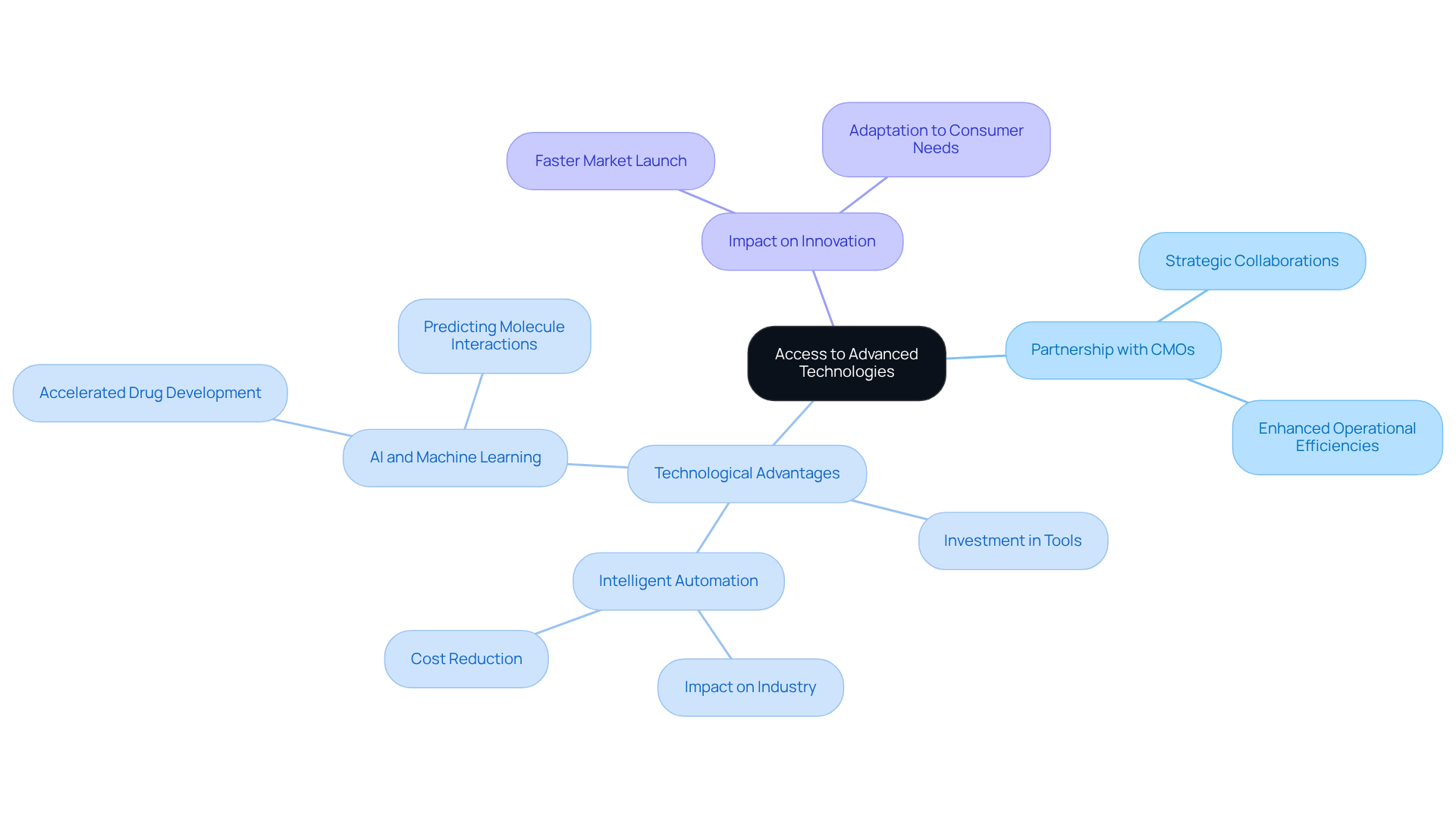
Access to Advanced Technologies: Leveraging Expertise for Product Innovation
Partnering with contract manufacturing organizations pharmaceutical provides pharmaceutical firms with access to advanced technologies and specialized knowledge that may not be feasible to develop internally. CMOs are committed to investing in cutting-edge tools and innovative methods, significantly enhancing their capacity to manufacture high-quality products efficiently. This technological advantage not only fosters innovation in products but also enables companies to adapt swiftly to the evolving needs of consumers.
By leveraging these advanced resources, businesses can sustain a competitive edge and expedite the launch of innovative products into the market. For instance, 79% of pharmaceutical executives believe that intelligent automation will have a significant impact on their industry in the next five years. Furthermore, the integration of AI and machine learning in chief marketing officers has streamlined processes, reduced costs, and improved the overall efficiency of drug development, allowing for faster responses to market demands.
Notably, AI can reduce drug development timelines from five years to as little as 12-18 months, illustrating how technology is transforming the industry. A case study highlighting AI's role in accelerating drug discovery further exemplifies the practical application of technology in contract manufacturing organizations pharmaceutical and its influence on innovation.
Accelerated Time to Market: Enhancing Speed with CDMO Partnerships
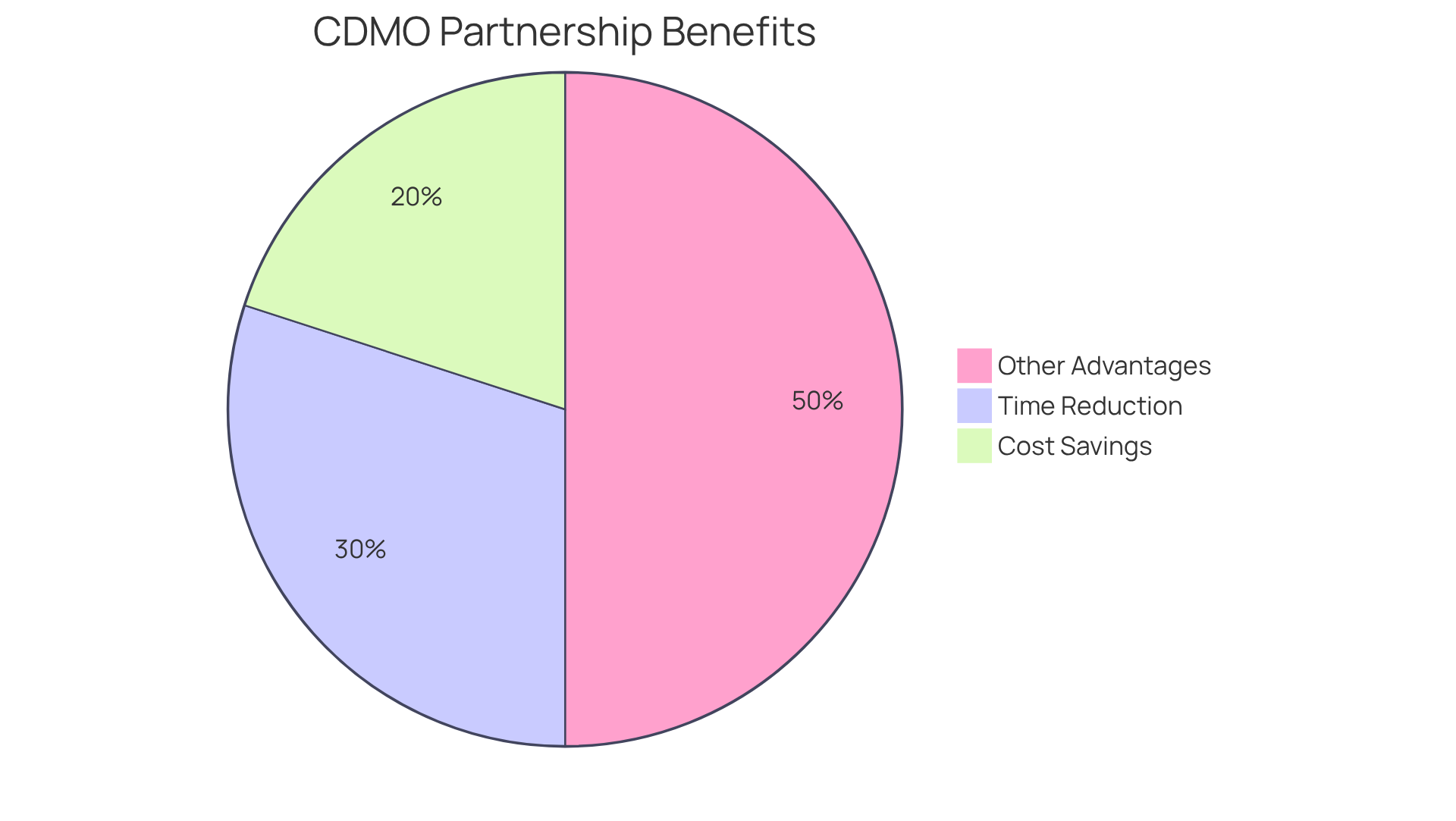
Collaborating with contract manufacturing organizations pharmaceutical significantly accelerates time to market for pharmaceutical firms. Contract manufacturing organizations pharmaceutical streamline the manufacturing process, which enables quicker product launches that are essential in a competitive landscape. In fact, organizations that leverage contract manufacturing organizations pharmaceutical partnerships can reduce development time by as much as 30%, allowing them to respond rapidly to consumer demand and capitalize on emerging trends. This speed not only enhances market presence but also boosts sales potential and market share.
For instance, during the COVID-19 pandemic, companies like BioNTech utilized CMO services to expedite mRNA vaccine production, illustrating how flexibility in collaborations can distinguish organizations from traditional drug manufacturers. As BioNTech stated, "The speed and agility afforded by our CMO partnerships were crucial in our ability to deliver vaccines swiftly during the pandemic."
Furthermore, a study by Bain & Company highlights that drug companies employing CDMOs can achieve lead time reductions of approximately 30%, underscoring the importance of speed in product launches. Additionally, Frost & Sullivan reports that businesses using CDMOs can reduce manufacturing costs by about 20-30%, further highlighting the financial advantages of these partnerships. As the industry continues to evolve, the ability to introduce offerings swiftly through collaboration with contract manufacturing organizations pharmaceutical remains a vital competitive edge.
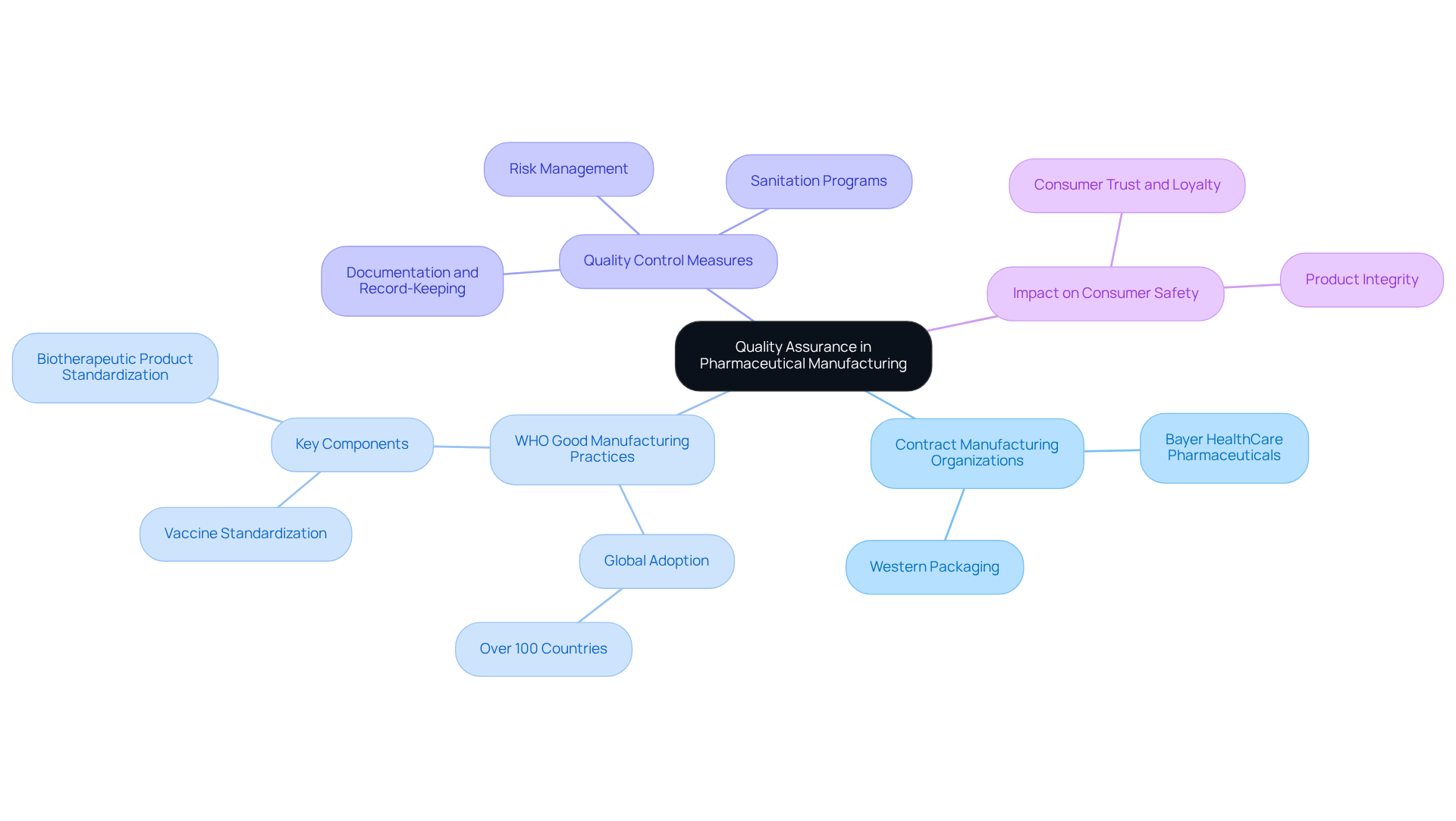
Quality Assurance: Ensuring High Standards in Pharmaceutical Manufacturing
Quality assurance stands as a cornerstone in drug manufacturing, with contract manufacturing organizations pharmaceutical like Western Packaging at the forefront of maintaining rigorous standards throughout the production process. These organizations implement comprehensive quality control measures, guaranteeing that every item not only adheres to regulatory requirements but also fulfills consumer expectations.
For instance, over 100 nations have adopted WHO Good Manufacturing Practices (GMP), which serve as a benchmark for quality in drug production. This unwavering commitment to quality not only protects consumers but also significantly bolsters brand reputation, fostering trust and loyalty among customers.
Quality control specialists emphasize that maintaining strict standards is vital for pharmaceutical contract manufacturing organizations, as it directly impacts item integrity and consumer safety. As one expert aptly stated, "Implementing a robust quality management system is crucial for mitigating risks and ensuring compliance in pharmaceutical production."
Moreover, case studies reveal that CMOs employing such systems, like Bayer HealthCare Pharmaceuticals, have adeptly navigated regulatory challenges while enhancing quality. Furthermore, the significance of documentation and record-keeping in GMP compliance cannot be overstated, as it ensures traceability and accountability in the manufacturing process.
By partnering with contract manufacturing organizations pharmaceutical, businesses can ensure their products consistently meet the highest quality standards, ultimately driving operational efficiency and achieving market success.
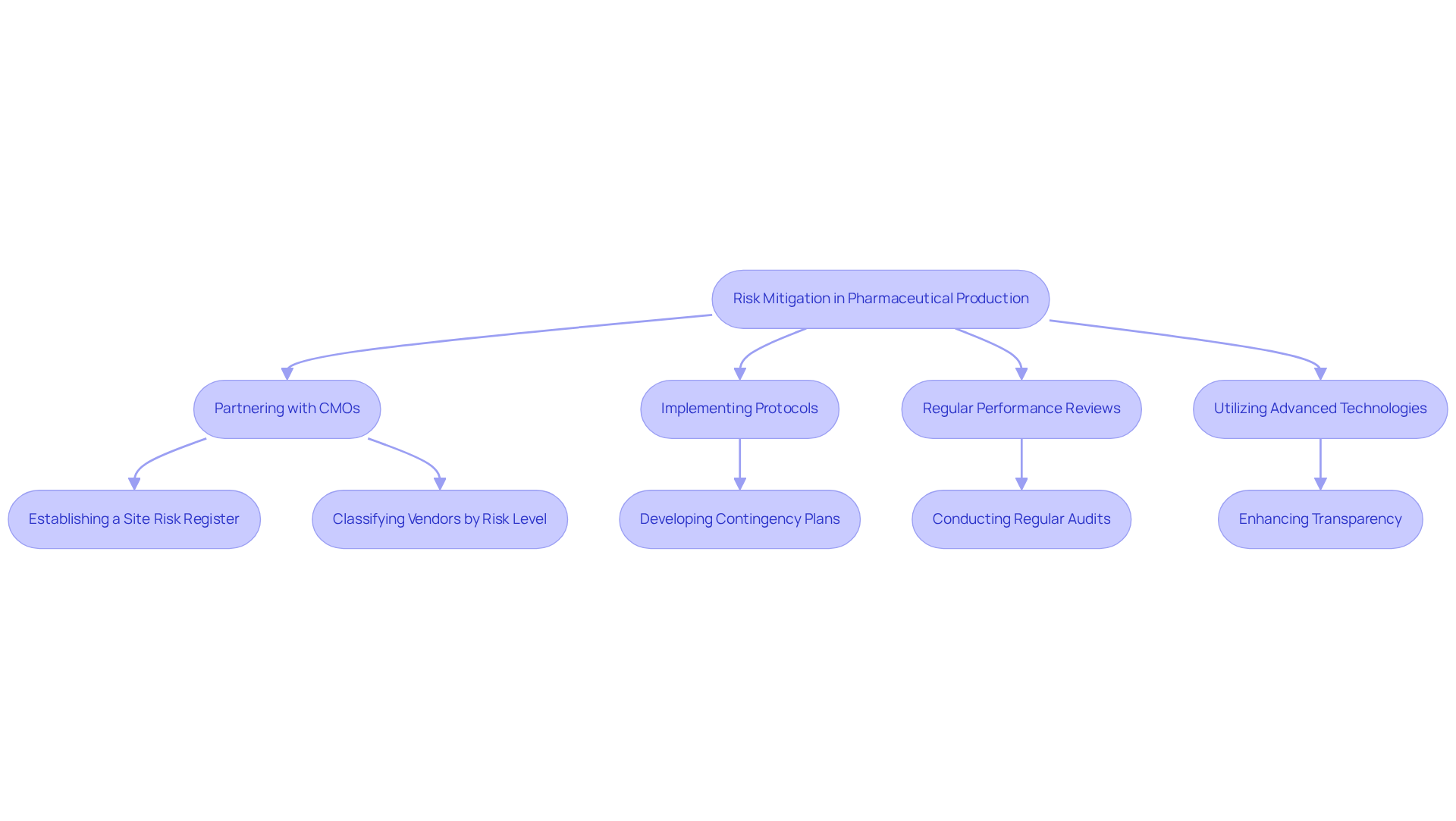
Risk Mitigation: Reducing Uncertainties in Pharmaceutical Production
Partnering with contract manufacturing organizations pharmaceutical represents a strategic decision for firms intent on minimizing production risks. Marketing executives deploy robust protocols and contingency plans specifically designed to address potential disruptions, such as supply chain challenges and regulatory shifts. This proactive risk management framework empowers companies to avert costly delays, thereby ensuring consistent adherence to production timelines.
By leveraging the expertise of a CMO, businesses can adeptly navigate uncertainties, safeguarding their investments and ensuring seamless operational continuity. Industry leaders underscore that effective communication and clear expectations with Chief Marketing Officers are essential for managing these risks. For instance, regular performance reviews and audits are instrumental in maintaining compliance and fostering a collaborative environment where potential issues can be swiftly identified and addressed.
Furthermore, Chief Marketing Officers frequently employ advanced technologies to enhance transparency and monitor supplier performance, a necessity in today's intricate regulatory landscape. Notably, 29% of organizations classify vendors by risk level, applying varying degrees of checks and precautions based on that assessment, which highlights the significance of tailored risk management strategies.
This integrated approach not only protects product integrity but also enhances overall efficiency in the production process, making contract manufacturing organizations pharmaceutical invaluable partners in the sector. As Wayne Back articulates, establishing a site risk register is a remarkable achievement for organizations, providing a singular document that encapsulates the complex multidepartmental effort of risk management. Continuous observation and assessment of risks are imperative to adapt to the ever-evolving environment, ensuring that organizations remain robust and compliant.
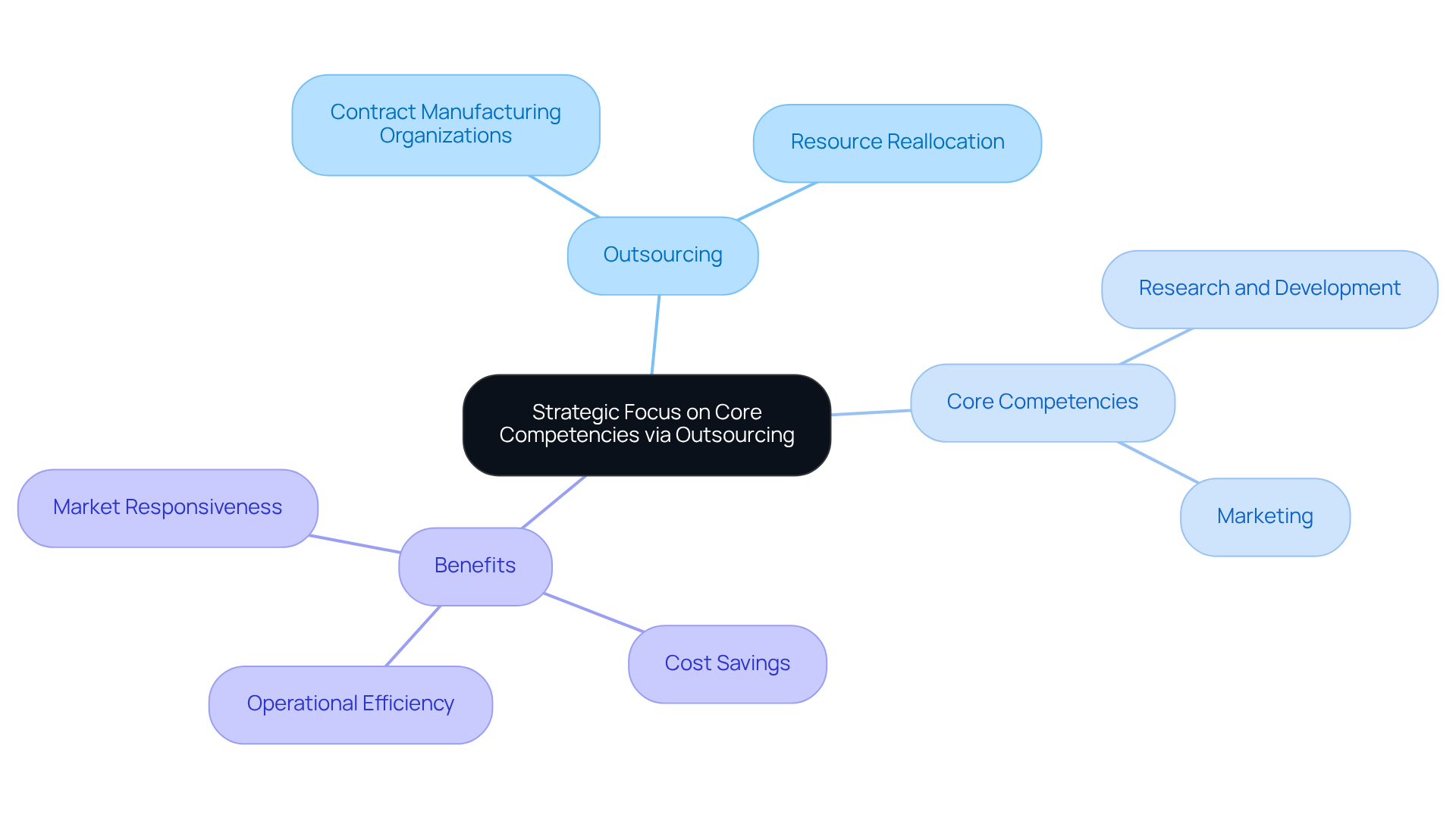
Strategic Focus: Concentrating on Core Competencies Through Outsourcing
Outsourcing production to contract manufacturing organizations pharmaceutical empowers pharmaceutical firms to concentrate on their core competencies, notably research and development and marketing. By delegating production to specialized partners, organizations can reallocate resources to growth-oriented areas, thereby fostering innovation and enhancing operational efficiency.
For example, SSB successfully reduced an organization's monthly burn rate from $300,000 to $30,000. This case illustrates how strategic outsourcing can yield substantial cost savings and sharpen operational focus. Such a strategic emphasis not only streamlines processes but also equips businesses to swiftly adapt to market demands and evolving consumer preferences.
As noted, 'Based in large part on the impact of SSB's efforts, the organization has enjoyed significant growth in recent years,' underscoring the tangible benefits of outsourcing. Numerous drug companies have effectively collaborated with contract manufacturing organizations pharmaceutical to enhance their capabilities, allowing them to maintain a competitive edge while refining their internal operations.
The impact of outsourcing is profound; it allows firms to concentrate on their strengths, ultimately leading to enhanced offerings and increased market responsiveness.
Innovation and Collaboration: Fostering New Product Development with CDMOs
Collaboration with contract manufacturing organizations pharmaceutical drives innovation and the development of new offerings in the pharmaceutical sector. By leveraging the expertise and resources of a CMO, companies can explore new formulations, packaging designs, and delivery methods that align with evolving consumer needs.
Western Packaging's integrated filling services connect seamlessly with customized flexible packaging solutions, streamlining production processes and enhancing product appeal. This collaborative approach not only enriches product offerings but also fosters market differentiation, enabling businesses to stand out in a competitive marketplace.
By working together, pharmaceutical companies and contract manufacturing organizations pharmaceutical can create innovative solutions that resonate with consumers and drive sales.
Conclusion
Partnering with contract manufacturing organizations (CMOs) in the pharmaceutical industry presents a wealth of advantages that can significantly boost operational efficiency and market competitiveness. By leveraging the expertise and resources of these specialized entities, pharmaceutical companies can adeptly navigate complex regulatory landscapes, reduce costs, and accelerate time to market. This strategic collaboration not only ensures compliance with stringent quality standards but also fosters innovation and agility in responding to market demands.
The article underscores several key benefits of engaging with CMOs, including:
- Cost-effectiveness through outsourcing
- Flexibility in production scaling
- Access to advanced technologies
- Robust risk mitigation strategies
Organizations that utilize these partnerships can concentrate on their core competencies, such as research and development, while CMOs manage the intricacies of manufacturing and compliance. This division of labor empowers companies to innovate more effectively and maintain a competitive edge in a rapidly evolving industry.
Ultimately, the importance of contract manufacturing organizations in the pharmaceutical sector cannot be overstated. As the industry continues to encounter challenges and opportunities, leveraging the strengths of CMOs will be crucial for companies aiming to enhance their operational capabilities and drive sustainable growth. Embracing these partnerships will not only streamline processes but also empower pharmaceutical firms to meet consumer needs more efficiently and effectively, securing their success in the marketplace.
Frequently Asked Questions
What services does Western Packaging offer for pharmaceutical companies?
Western Packaging offers integrated packaging solutions that include packaging design, customized filling services, and extensive third-party logistics (3PL) to streamline the pharmaceutical packaging process.
How does Western Packaging enhance efficiency in the pharmaceutical supply chain?
They create a seamless workflow that reduces lead times and enhances delivery efficiency through their end-to-end solutions, which also include warehousing and inventory management.
What is the role of contract manufacturing organizations (CMOs) like Western Packaging in regulatory compliance?
CMOs help navigate the complex regulatory landscape of the pharmaceutical industry by ensuring that all packaging and manufacturing processes adhere to FDA regulations and compliance standards.
How do CMOs protect companies from legal issues in the pharmaceutical sector?
Their understanding of compliance standards shields companies from potential legal issues and fosters consumer confidence in their products.
What financial benefits can pharmaceutical companies gain from outsourcing manufacturing to CMOs?
Outsourcing can lead to substantial reductions in operational costs, with potential savings of up to 30% on production expenses, allowing companies to reallocate resources more efficiently.
How do CMOs contribute to production flexibility for pharmaceutical companies?
CMOs allow organizations to rapidly increase production in response to market needs without the burden of fixed expenses, making them vital partners in the healthcare industry.
What impact does partnering with CMOs have on product development and marketing strategies?
By tackling compliance challenges, companies can focus more on product development and marketing strategies, enhancing their overall operational efficiency.
How do established supply chains of CMOs help pharmaceutical companies?
CMOs typically have established supply chains and strong relationships with suppliers, which contribute to cost reductions and improved production efficiency.




