Overview
The article titled "7 Key Benefits of Contract Manufacturing in Pharmaceuticals" delves into the significant advantages that contract manufacturing presents to pharmaceutical companies. It highlights key benefits, including:
- Cost-effectiveness
- Regulatory compliance
- Scalability
- Access to expertise
- Flexibility
- Risk mitigation
- Faster time-to-market
These factors collectively enhance operational efficiency and market competitiveness for drug manufacturers, establishing a compelling case for the adoption of contract manufacturing solutions.
Introduction
The pharmaceutical industry is experiencing a transformative shift as companies increasingly embrace contract manufacturing to enhance efficiency and reduce costs. This strategic approach not only streamlines production but also offers a multitude of benefits, ranging from regulatory compliance to accelerated time-to-market for new products.
What, then, are the key advantages that position contract manufacturing as an essential component in modern pharmaceutical operations? By exploring these benefits, we uncover how this collaboration empowers companies to navigate challenges while driving innovation and operational excellence.
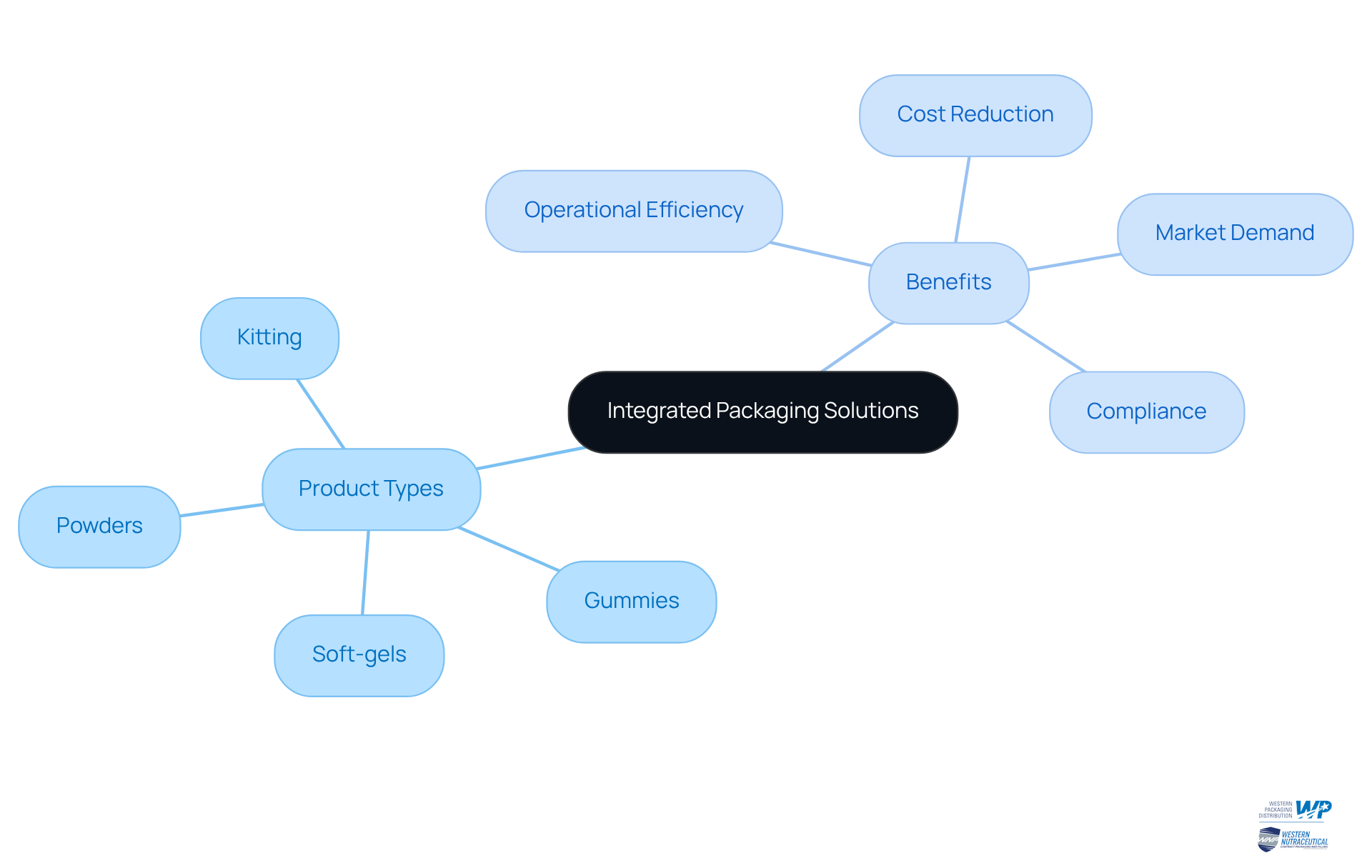
Western Packaging: Integrated Packaging Solutions for Enhanced Efficiency
Western Packaging offers a robust suite of integrated packaging solutions that seamlessly combine design, filling, and third-party logistics (3PL) services. Our filling process in contract manufacturing pharmaceuticals accommodates a wide range of products, including:
- Powders
- Gummies
- Soft-gels
- Kitting
This ensures that companies in the healthcare sector can optimize their supply chains effectively. This comprehensive approach empowers clients to significantly reduce lead times and enhance product delivery. By leveraging innovative packaging designs and efficient filling processes in contract manufacturing pharmaceuticals, Western Packaging elevates product appeal while ensuring strict compliance with industry standards, including FDA regulations and tamper-evident packaging requirements. Our proficiency in flexible packaging solutions enables clients to effectively address diverse market demands, ultimately driving operational efficiency and improving overall supply chain performance. Statistics indicate that companies implementing integrated packaging and logistics strategies can achieve up to a 30% decrease in operational expenses, underscoring the significance of this comprehensive approach in the healthcare industry.
Regulatory Compliance: Ensuring Safety and Quality in Pharmaceuticals
In the drug industry, regulatory compliance is paramount for ensuring that products adhere to stringent safety and quality standards. Contract manufacturing pharmaceuticals play a pivotal role in this landscape, as they typically possess established systems and processes designed to navigate the complex regulatory environment. By partnering with skilled producers in contract manufacturing pharmaceuticals, companies can effectively ensure compliance with Good Manufacturing Practices (GMP) and other regulatory guidelines. This partnership not only safeguards consumer health but also enhances brand reputation.
The significance of GMP in contractual production cannot be overstated. These practices are specifically designed to prevent contamination, mix-ups, and deviations during the manufacturing process, thereby ensuring the identity, strength, quality, and purity of drug products. Production firms apply strict quality assurance practices, which involve detailed documentation and oversight of active substances to ensure they are devoid of impurities and satisfy the necessary concentrations.
Moreover, the impact of GMP compliance on pharmaceutical safety is profound. By adhering to these standards, production companies help reduce risks associated with drug creation, ultimately safeguarding public health. This commitment to quality fulfills not only regulatory obligations but also positions companies as trustworthy providers in a competitive market.
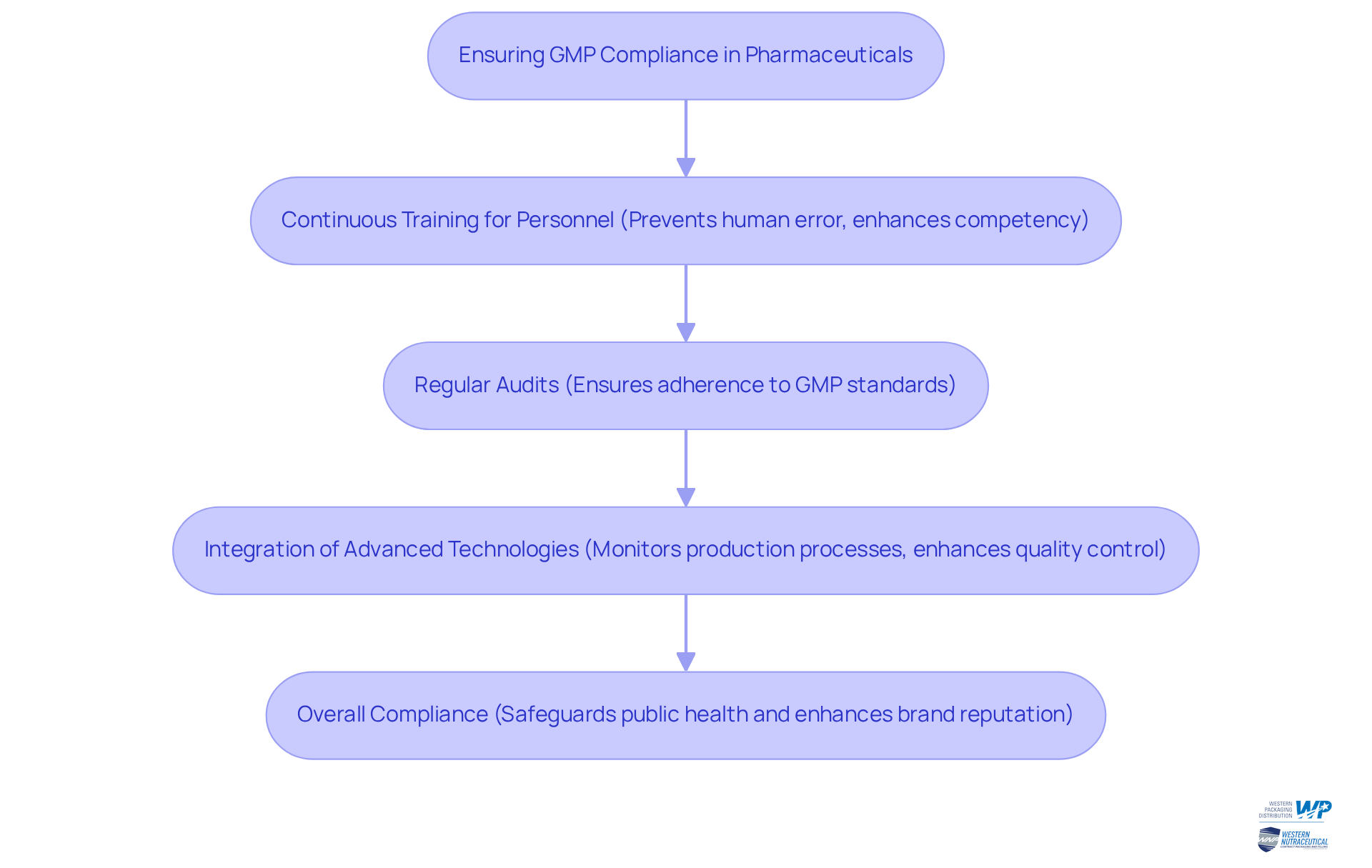
To ensure GMP compliance, companies involved in contract manufacturing pharmaceuticals utilize a variety of strategies, including:
- Continuous training for personnel
- Regular audits
- The integration of advanced technologies to monitor production processes
Notably, human error accounts for 40% to 65% of serious GMP deviations, highlighting the critical need for effective training programs. By fostering a culture of quality and adherence, these producers can swiftly adapt to changing regulatory demands, ensuring that their clients remain competitive and compliant in a fast-evolving industry.
The historical impact of GMP on drug safety is underscored by events such as the thalidomide tragedy, which emphasized the necessity of stringent regulations to prevent similar occurrences. As regulatory bodies continue to enforce compliance through inspections and audits, the role of pharmacovigilance becomes increasingly important in monitoring long-term drug effects and ensuring ongoing safety. By prioritizing GMP, contract manufacturing pharmaceuticals not only enhances product quality but also contributes to the overall integrity of the drug supply chain.
Cost-Effectiveness: Reducing Operational Expenses through Contract Manufacturing
Contract manufacturing pharmaceuticals offers a significant advantage in cost efficiency, especially for pharmaceutical companies. By outsourcing production, these companies can circumvent the significant capital expenditures linked to the establishment and upkeep of production facilities.
Contract producers leverage economies of scale, enabling them to manufacture medicines at markedly reduced costs. This decrease in operational expenses not only permits companies to allocate resources more effectively but also empowers them to invest more heavily in research and development. Consequently, businesses can bolster their market competitiveness and enhance their agility in responding to industry demands.
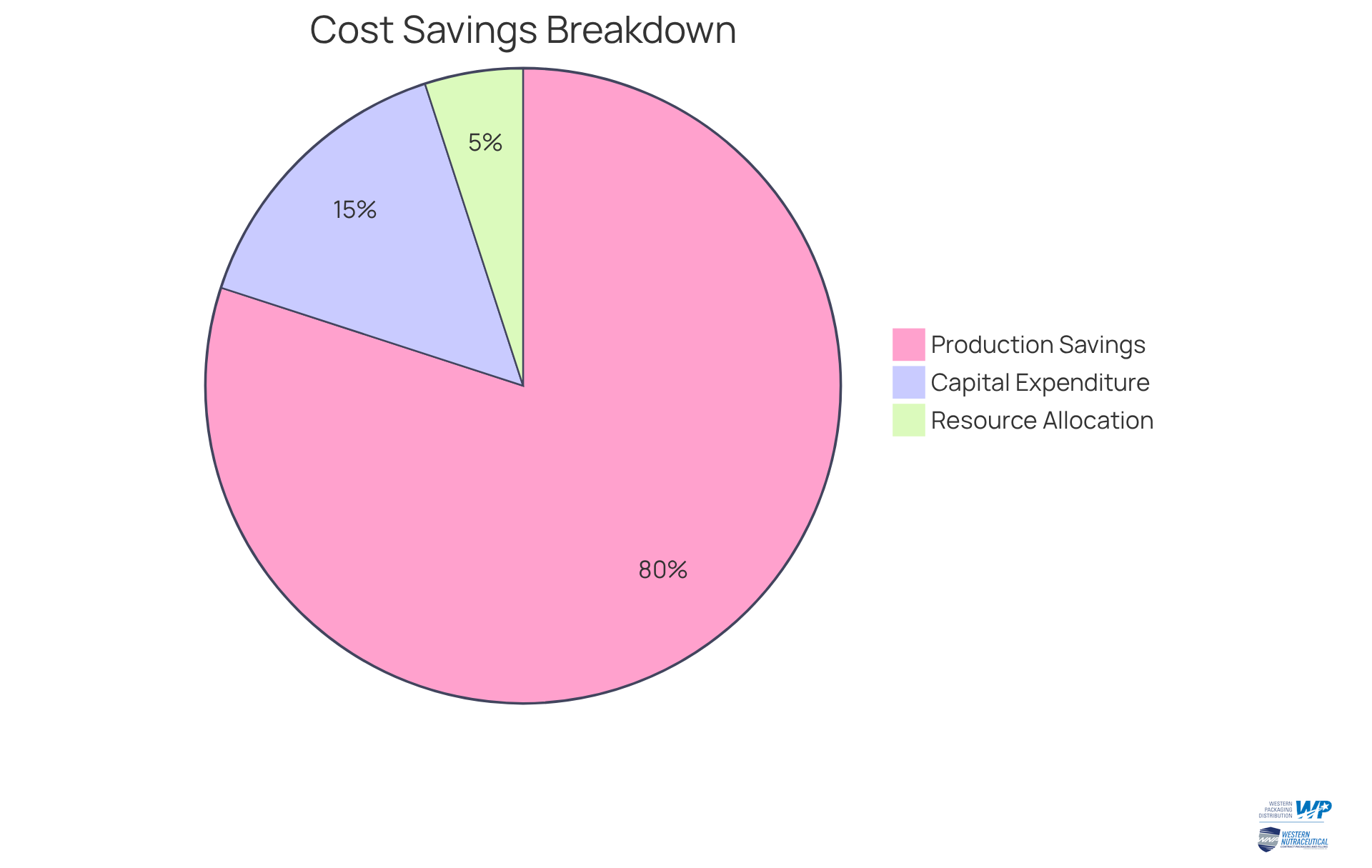
Notably, drug producers can achieve savings of up to 80% in production expenses through contract manufacturing pharmaceuticals by partnering with contract manufacturing organizations (CMOs), underscoring the profound impact of outsourcing on operational efficiency. Furthermore, the average savings in capital expenditures through contract manufacturing pharmaceuticals can reach substantial figures, highlighting the strategic value of this approach within the industry.
Scalability: Adapting Production Levels to Market Demand
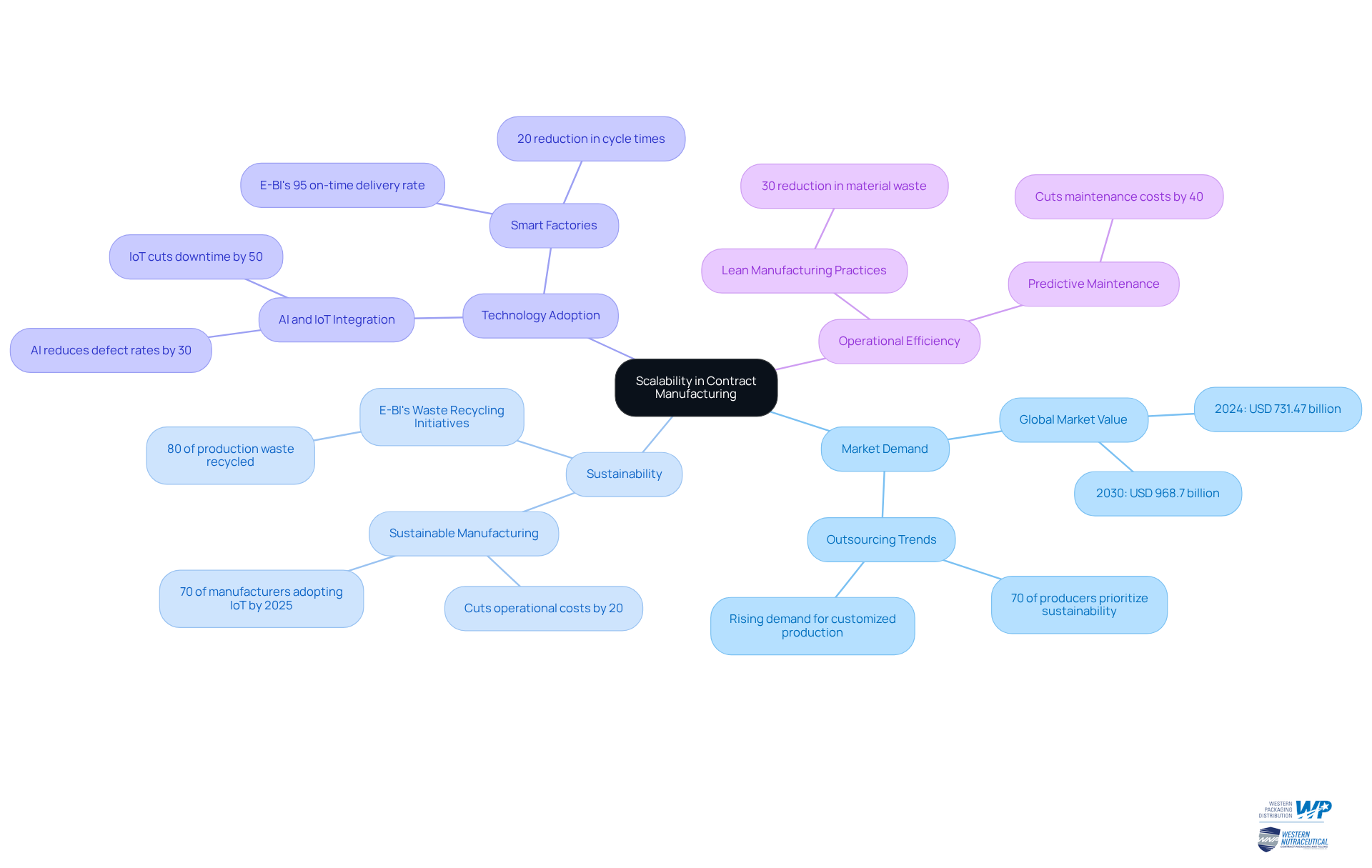
Contract manufacturing pharmaceuticals offers unparalleled scalability, empowering pharmaceutical firms to adjust output levels in response to market demand. This adaptability is particularly advantageous in an industry marked by swift shifts in consumer preferences and regulatory changes. By partnering with production firms, companies can rapidly increase output for new product launches or reduce it during periods of diminished demand, thereby enhancing inventory management and minimizing waste.
The global contract production market, valued at USD 731.47 billion in 2024, is projected to reach approximately USD 968.7 billion by 2030, highlighting the evident demand for flexible production capabilities. Businesses are increasingly outsourcing production to bolster their responsiveness to market fluctuations, with 70% of producers prioritizing sustainability in their outsourcing decisions. Furthermore, by 2025, 70% of producers are expected to adopt IoT for real-time monitoring, underscoring the technological evolution alongside sustainability efforts.
Moreover, the incorporation of advanced technologies, such as AI and IoT, allows manufacturers to refine production processes, further enhancing their capacity to meet market demands. For instance, E-BI's smart factories in Thailand have achieved an impressive 95% on-time delivery rate for electronics OEMs through the utilization of IoT technology, illustrating how operational efficiency can be significantly improved through strategic production partnerships. Additionally, AI in manufacturing is shown to reduce defect rates by 30%, showcasing the benefits of advanced technologies in production.
In conclusion, the ability of production firms to adjust output levels not only aids drug companies in fulfilling market needs but also fosters innovation and sustainability within contract manufacturing pharmaceuticals.
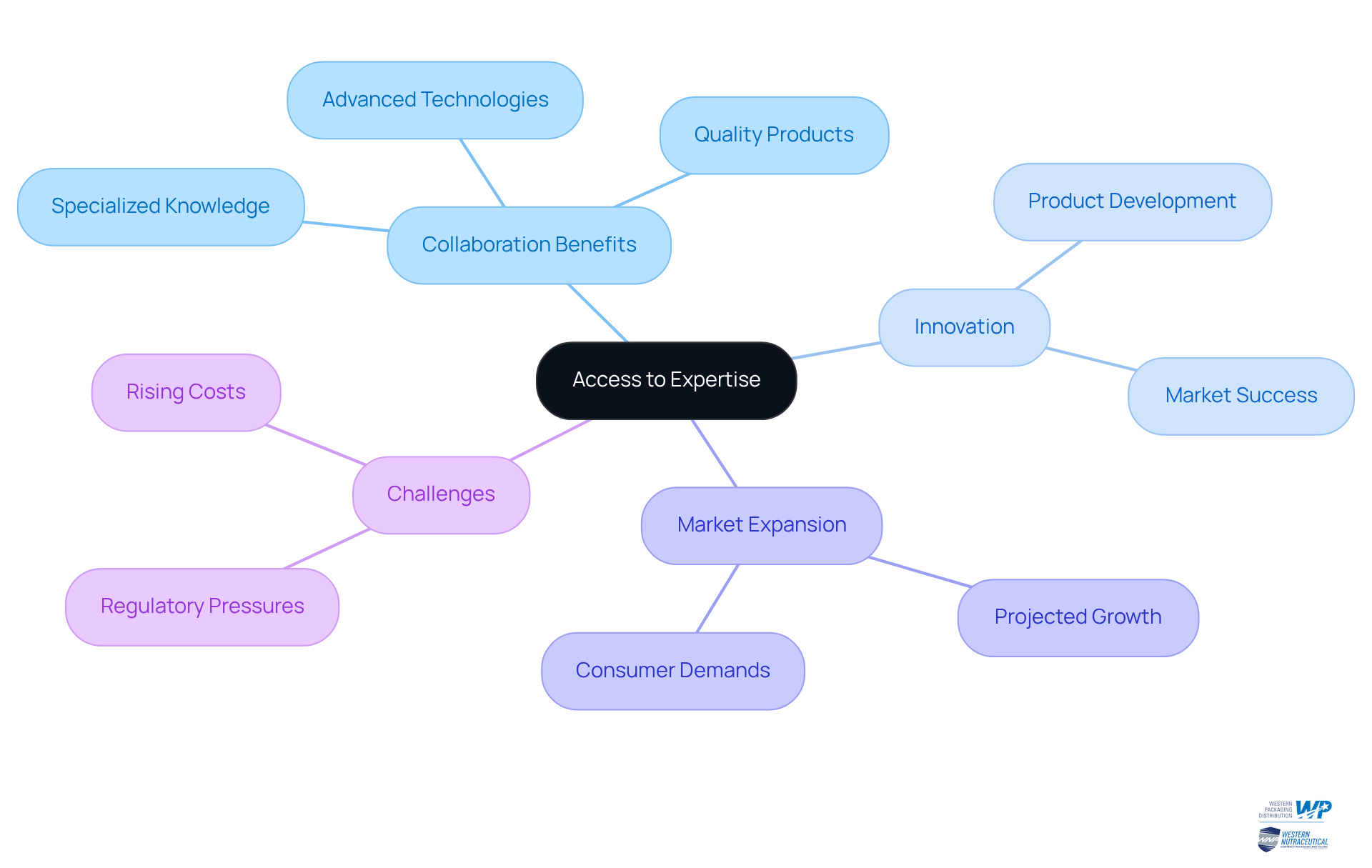
Access to Expertise: Leveraging Advanced Technology for Product Innovation
Collaborating with production partners empowers drug companies to tap into specialized knowledge and advanced technologies that may not be available internally. These producers often lead the way in innovation, investing in state-of-the-art equipment and processes that facilitate the efficient production of high-quality products. This strategic alliance not only accelerates product innovation but also enhances formulations and overall product quality. Consequently, companies can achieve greater market success and respond more effectively to consumer demands.
The essence of collaboration is encapsulated in the words of Steve Jobs, who remarked that innovation is about perceiving change as an opportunity. By leveraging the strengths of manufacturing partners in contract manufacturing pharmaceuticals, pharmaceutical companies can navigate the complexities of product development and maintain a competitive edge in a rapidly evolving market.
Furthermore, the Global Contract Manufacturing Pharmaceuticals Market is projected to expand to USD 1,256.57 billion by 2032, highlighting the growing significance of these partnerships. However, it is crucial to recognize the challenges faced by the production industry, such as rising costs and regulatory pressures, which necessitate strong relationships for future opportunities.
Flexibility: Customizing Production Processes for Unique Requirements
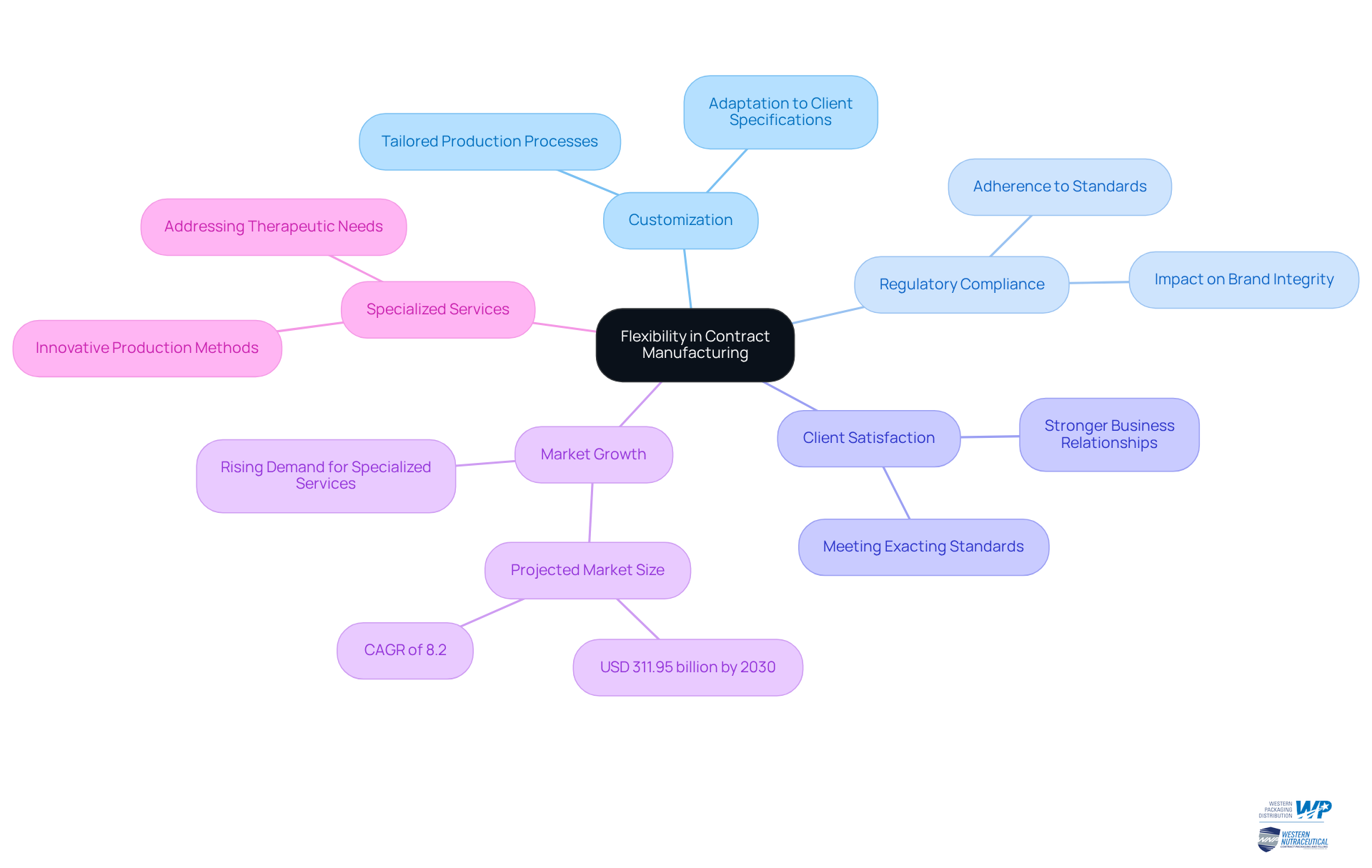
Flexibility stands as a defining characteristic of contract manufacturing, empowering companies to customize production processes to meet specific product requirements. In the pharmaceutical industry, where contract manufacturing pharmaceuticals involves precision in formulations, packaging, and labeling, this adaptability proves crucial. Contract manufacturers possess the capability to modify their processes in alignment with unique client specifications, thereby ensuring compliance with regulatory standards and enhancing brand integrity.
The growing intricacy of injectable drug formats necessitates innovative production methods, enabling customized solutions that effectively address various therapeutic needs. This level of customization not only elevates client satisfaction but also cultivates stronger business relationships, as companies can depend on their partners to deliver products that meet their exacting standards.
With the market for contract manufacturing pharmaceuticals projected to expand considerably, reaching USD 311.95 billion by 2030 with a CAGR of 8.2%, the ability to tailor production processes remains a vital competitive edge. Furthermore, the rising demand for specialized services underscores the importance of bespoke production processes in fulfilling market needs.
Focus on Core Competencies: Streamlining Operations for Greater Efficiency
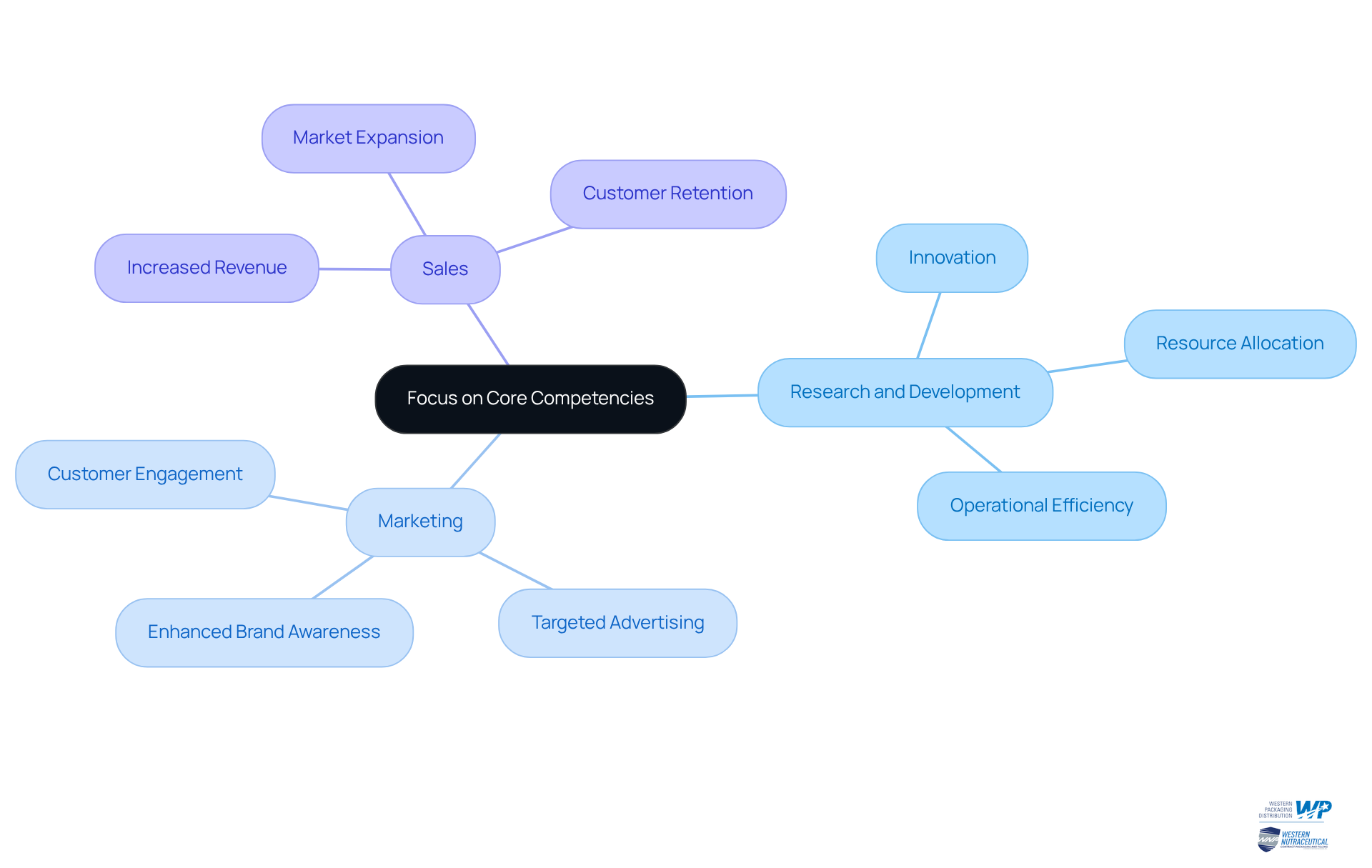
By engaging in contract manufacturing pharmaceuticals, drug companies can concentrate on their core strengths: research and development, marketing, and sales. This strategic focus not only allows companies to allocate resources more effectively but also drives innovation within their primary areas of expertise. As a result, companies can significantly enhance their operational efficiency, improve product quality, and ultimately achieve superior business outcomes.
Risk Mitigation: Managing Supply Chain Disruptions and Quality Control
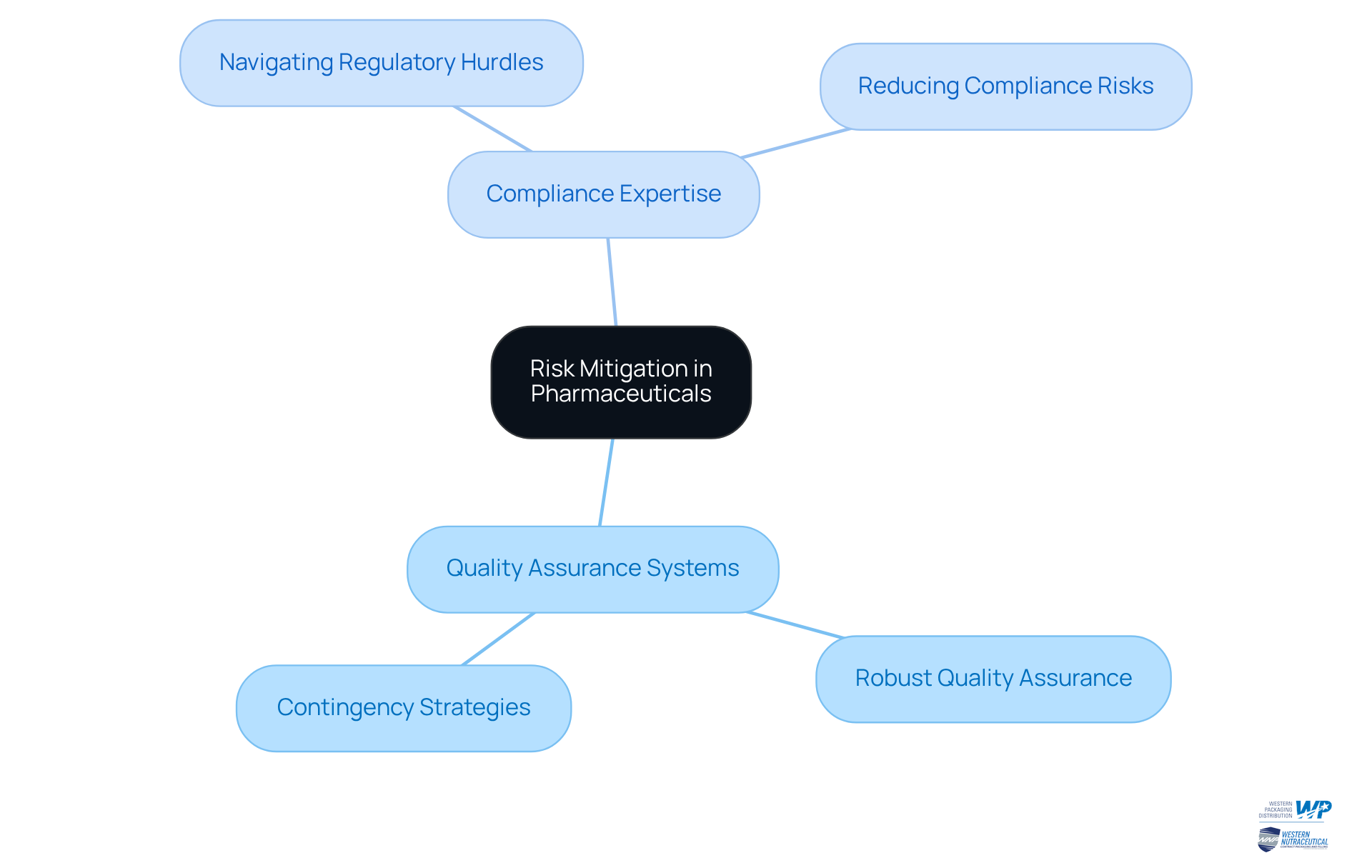
Contract manufacturing pharmaceuticals serves as a crucial mechanism for risk mitigation, effectively providing a buffer against supply chain disruptions. By partnering with reputable producers in contract manufacturing pharmaceuticals, drug companies can take advantage of their robust quality assurance systems and contingency strategies. This proactive stance guarantees uninterrupted production, even when confronted with unforeseen challenges.
Furthermore, production partners often bring specialized expertise in navigating regulatory hurdles, significantly reducing the risk of compliance issues. In an industry where reliability is paramount, choosing the right partner for contract manufacturing pharmaceuticals is not just an option; it is a necessity.
Faster Time-to-Market: Accelerating Product Launches in Pharmaceuticals
Contract manufacturing pharmaceuticals provides a critical advantage in accelerating the time-to-market for new medical products. By outsourcing production, companies can streamline their development processes and tap into the specialized expertise of manufacturers, significantly reducing product launch timelines. This speed is vital in the fiercely competitive pharmaceutical landscape, where contract manufacturing pharmaceuticals can enable companies to be first to market, yielding substantial benefits in market share and brand recognition.
Firms that have embraced outsourced production have reported a considerable reduction in product development timelines, enabling them to respond swiftly to market demands. The global market for contract manufacturing pharmaceuticals is projected to grow at a compound annual growth rate (CAGR) of 8.70%, reaching USD 1.12 trillion by 2030. This growth underscores the increasing reliance on contract manufacturing pharmaceuticals services to enhance operational efficiency and maintain competitiveness in the pharmaceutical sector.
Moreover, the integration of advanced technologies such as automation and predictive analytics within production processes allows for real-time monitoring and quality control, further expediting product readiness. As highlighted in industry insights, 'As product lifecycles shorten and competitive pressures to innovate intensify, brands increasingly turn to external manufacturers for expedited prototyping, mass production, and adaptable supply chain management.'
Instances of expedited product introductions abound, particularly in the healthcare industry, where companies have effectively leveraged contract manufacturing pharmaceuticals to launch innovative treatments ahead of competitors. For instance, various drug manufacturers have utilized contract manufacturing pharmaceuticals to hasten the introduction of new vaccines and therapies, demonstrating how outsourcing can significantly enhance speed and effectiveness. This trend not only accelerates product introductions but also enables health sector companies to concentrate on their core competencies, ultimately driving growth and profitability in a rapidly evolving industry.
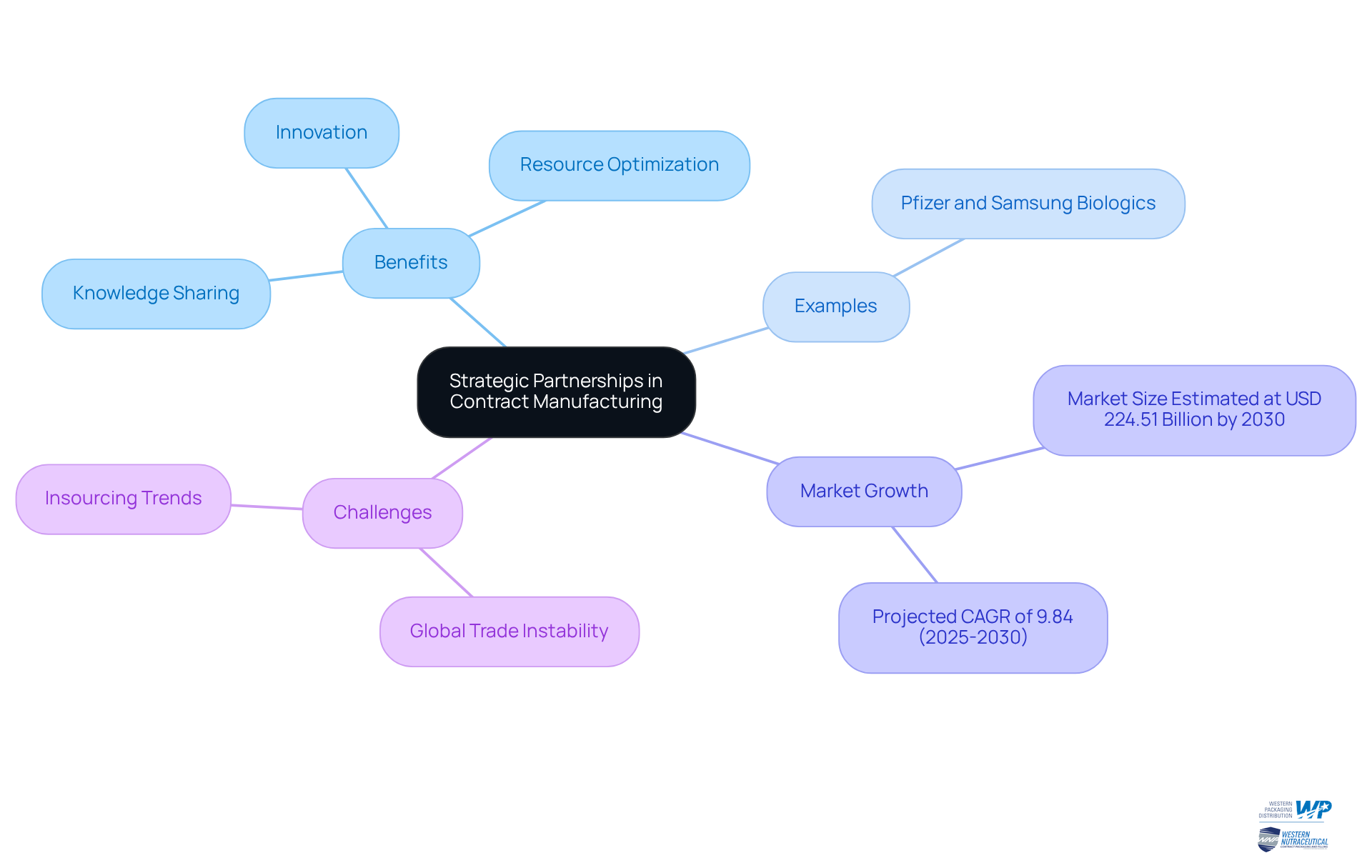
Strategic Partnerships: Enhancing Collaboration through Contract Manufacturing
Participating in contract manufacturing pharmaceuticals fosters strategic alliances that enhance cooperation between drug companies and producers. These alliances allow companies to share knowledge, resources, and best practices, culminating in improved operational capabilities and innovation. By collaborating closely with manufacturing partners, drug companies gain access to new technologies, optimize processes, and ultimately deliver superior products to market. This collaborative approach not only fortifies business relationships but also propels industry advancements.
For instance, Pfizer's agreement for production with Samsung Biologics, valued at USD 183 million, exemplifies the potential of such collaborations. As the contract manufacturing pharmaceuticals market is projected to grow at a CAGR of 9.84% from 2025 to 2030, the focus on collaboration becomes increasingly vital for success. However, challenges such as global trade instability and insourcing trends must also be navigated to fully harness the benefits of these partnerships.
As Janine Ogg & Jo Foster aptly stated, 'Just one great partnership can transform your entire business.
Conclusion
The advantages of contract manufacturing in the pharmaceutical industry are profound, providing companies with the opportunity to enhance efficiency, reduce costs, and ensure compliance with stringent regulatory standards. By leveraging the expertise of specialized production partners, pharmaceutical firms can focus on their core competencies while benefiting from scalable, flexible, and innovative manufacturing solutions. This strategic approach not only accelerates product launches but also strengthens brand integrity and fosters long-term partnerships that drive industry advancements.
Key insights from the article highlight the multifaceted benefits of contract manufacturing, including:
- Cost-effectiveness through reduced operational expenses
- The critical importance of regulatory compliance in safeguarding public health
- The ability to swiftly adapt production levels to meet changing market demands
Furthermore, the emphasis on risk mitigation and quality control underscores the pivotal role that contract manufacturers play in maintaining the integrity of the pharmaceutical supply chain.
Ultimately, embracing contract manufacturing transcends a mere tactical decision; it stands as a strategic imperative for pharmaceutical companies aiming to thrive in a competitive landscape. By prioritizing collaboration, investing in advanced technologies, and fostering a culture of innovation, companies can position themselves for success in an evolving market. The future of pharmaceuticals will increasingly rely on these strategic partnerships, making it essential for businesses to understand and leverage the myriad benefits that contract manufacturing offers.
Frequently Asked Questions
What integrated packaging solutions does Western Packaging offer?
Western Packaging provides a suite of integrated packaging solutions that include design, filling, and third-party logistics (3PL) services, catering to a variety of products such as powders, gummies, soft-gels, and kitting.
How does Western Packaging help companies in the healthcare sector?
Western Packaging helps healthcare companies optimize their supply chains by reducing lead times and enhancing product delivery through innovative packaging designs and efficient filling processes, while ensuring compliance with industry standards.
What are the benefits of implementing integrated packaging and logistics strategies?
Companies that implement integrated packaging and logistics strategies can achieve up to a 30% decrease in operational expenses, highlighting the effectiveness of this comprehensive approach in the healthcare industry.
Why is regulatory compliance important in the pharmaceutical industry?
Regulatory compliance is crucial in the pharmaceutical industry to ensure that products meet stringent safety and quality standards, protecting consumer health and enhancing brand reputation.
What role does contract manufacturing play in regulatory compliance?
Contract manufacturing is vital as it provides established systems and processes that help companies navigate the complex regulatory environment and ensure compliance with Good Manufacturing Practices (GMP) and other guidelines.
What is the significance of Good Manufacturing Practices (GMP)?
GMP practices are designed to prevent contamination, mix-ups, and deviations during the manufacturing process, ensuring the identity, strength, quality, and purity of drug products, thus safeguarding public health.
What strategies do companies use to ensure GMP compliance?
Companies ensure GMP compliance through continuous training for personnel, regular audits, and the integration of advanced technologies to monitor production processes.
How does contract manufacturing contribute to cost-effectiveness in pharmaceuticals?
Contract manufacturing reduces operational expenses by allowing pharmaceutical companies to outsource production, avoiding significant capital expenditures associated with production facilities, and leveraging economies of scale.
What potential savings can drug producers achieve through contract manufacturing?
Drug producers can achieve savings of up to 80% in production expenses by partnering with contract manufacturing organizations (CMOs), significantly impacting operational efficiency.




