Overview
This article delves into the optimization of pharmaceutical cold chain management, highlighting various strategies that uphold the integrity of temperature-sensitive products. It underscores the critical role of integrated solutions and advanced monitoring technologies, alongside regulatory compliance and stakeholder collaboration. These elements are essential for enhancing operational efficiency and mitigating the risks posed by temperature fluctuations. Ultimately, this approach supports the safe delivery of pharmaceuticals, reinforcing the need for reliable practices in the industry.
Introduction
The pharmaceutical industry stands at a critical juncture, where the integrity of temperature-sensitive products can mean the difference between life and death. With the surging demand for biologics and personalized medicine, effective cold chain management has never been more essential. This article explores seven key strategies designed to optimize pharmaceutical cold chain logistics. These strategies not only ensure compliance but also enhance product efficacy and minimize costly errors.
How can companies effectively navigate the complexities of regulatory requirements while adopting innovative technologies to safeguard their products? The answers lie within these strategies, which promise to elevate cold chain operations to unprecedented heights.
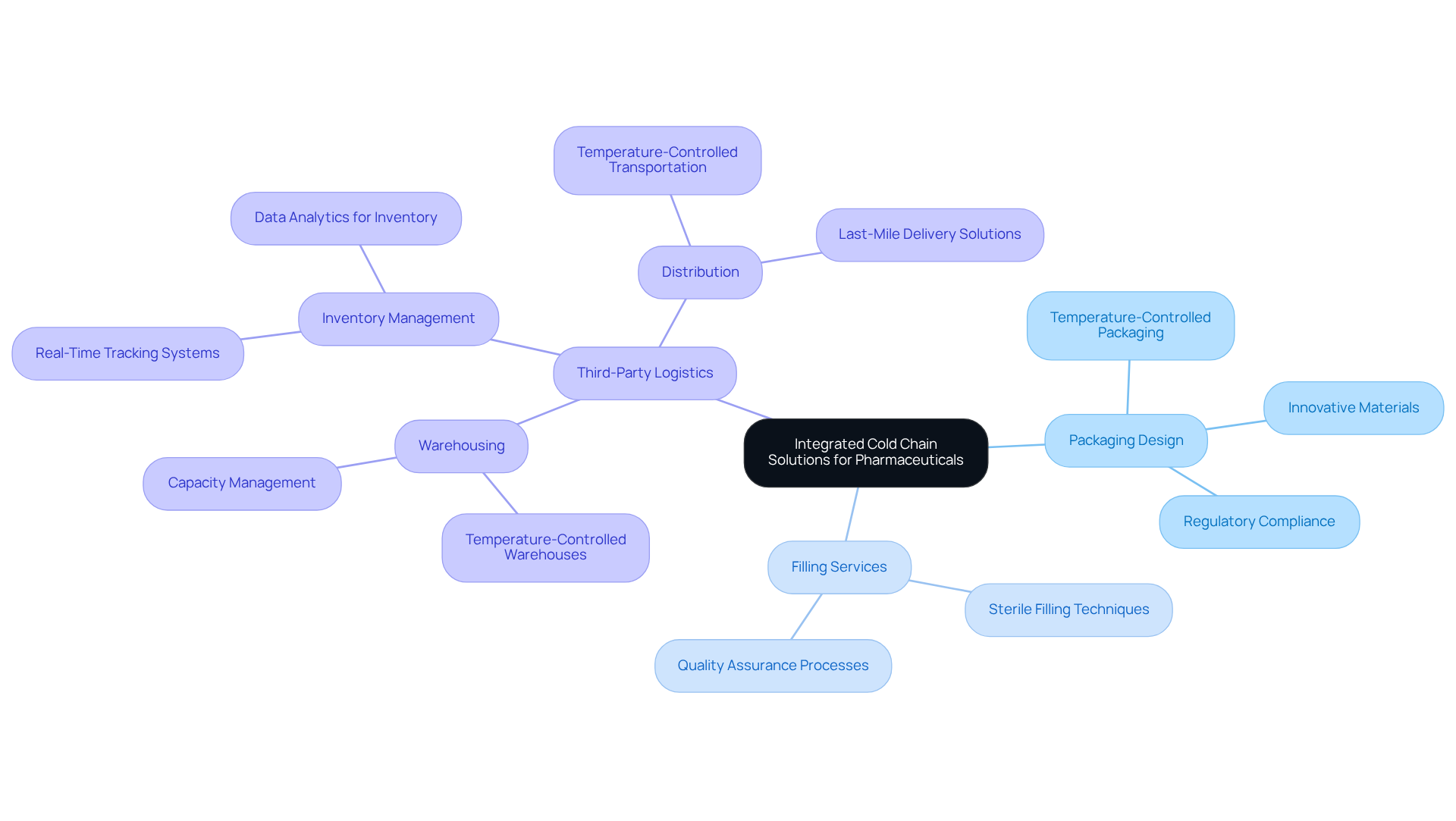
Western Packaging: Integrated Cold Chain Solutions for Pharmaceuticals
Western Packaging presents a distinctive integrated strategy for cold storage solutions that cater specifically to the pharmaceutical cold chain in the healthcare industry. By combining expert packaging design, filling services, and comprehensive third-party logistics (3PL)—including warehousing, inventory management, and distribution—the company guarantees that temperature-sensitive products are handled with the utmost care. This holistic approach not only streamlines operations but also significantly enhances product integrity throughout the supply network. Consequently, drug manufacturers can more effectively comply with regulatory requirements and preserve product efficacy.
Successful case studies illustrate the benefits of this integrated strategy. For instance, firms that have adopted hybrid temperature management solutions—merging active and passive control—have reported improved temperature regulation for a wide range of sensitive products. The global refrigerated logistics market is projected to grow at a CAGR of 13% from 2024 to 2032, highlighting the increasing demand for efficient and reliable solutions in the pharmaceutical cold chain.
Industry leaders emphasize the importance of comprehensive strategies for the pharmaceutical cold chain that are temperature-controlled. With over 85% of biologics requiring temperature-controlled management, the synergy of packaging, filling, and logistics is vital for ensuring the secure delivery of these products. Furthermore, advancements in technology, such as real-time monitoring and data recorders, enhance operational efficiency and safety during transport, establishing integrated temperature-controlled management as an essential component of modern supply networks.
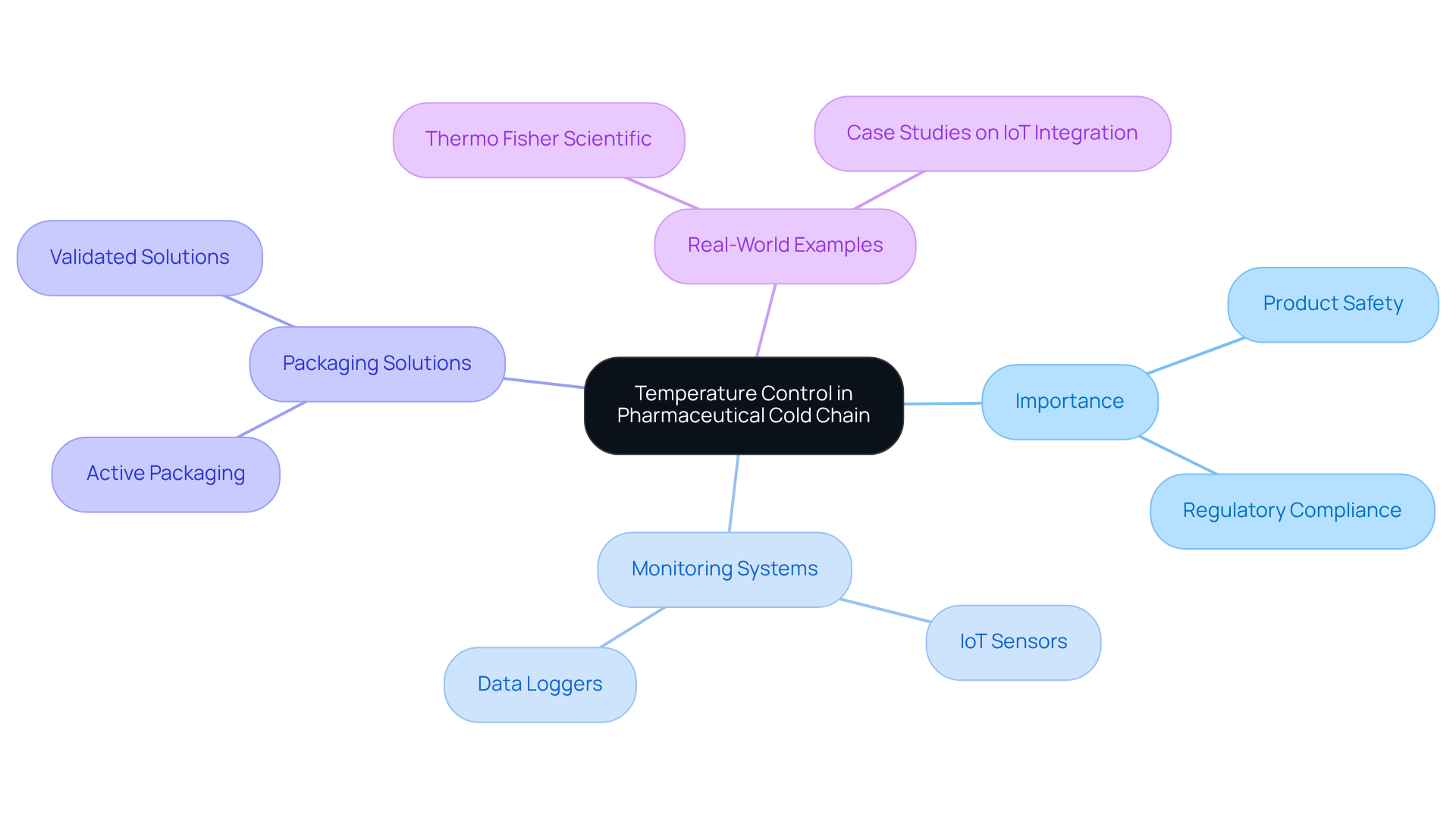
Temperature Control: The Backbone of Pharmaceutical Cold Chain Integrity
Regulating heat is essential in cold chain management for medications, as even minor fluctuations can endanger product safety and effectiveness. For numerous pharmaceuticals, maintaining a stable range between 2°C and 8°C is crucial. Experts highlight that advanced thermal monitoring systems, such as IoT sensors and data loggers, are vital for ensuring products stay within these critical thresholds throughout their journey from manufacturer to end-user. These systems provide real-time information, enabling proactive responses to any heat changes that may arise during storage and transportation.
Optimal methods for ensuring thermal integrity involve adopting robust monitoring protocols and utilizing validated packaging solutions designed for sensitive products. For instance, businesses are increasingly embracing active packaging formats, which lead the market due to their reliability and real-time climate control features. In practice, organizations such as Thermo Fisher Scientific employ integrated temperature management solutions to ensure that temperature-sensitive therapies are handled with the utmost care.
Real-world examples illustrate the effectiveness of these systems. The integration of IoT technology facilitates continuous tracking of environmental conditions, significantly reducing spoilage and ensuring compliance with regulatory standards. As the demand for biologics and personalized medicine escalates, the importance of preserving thermal integrity in pharmaceutical logistics becomes even more critical, fostering innovation and investment in advanced monitoring solutions. Notably, almost 50% of vaccines are lost each year due to inadequate temperature control, underscoring the necessity for efficient refrigeration solutions. Furthermore, the biologics market is projected to grow at a compound annual growth rate of 15% until 2027, highlighting the increasing significance of robust temperature-controlled management practices.
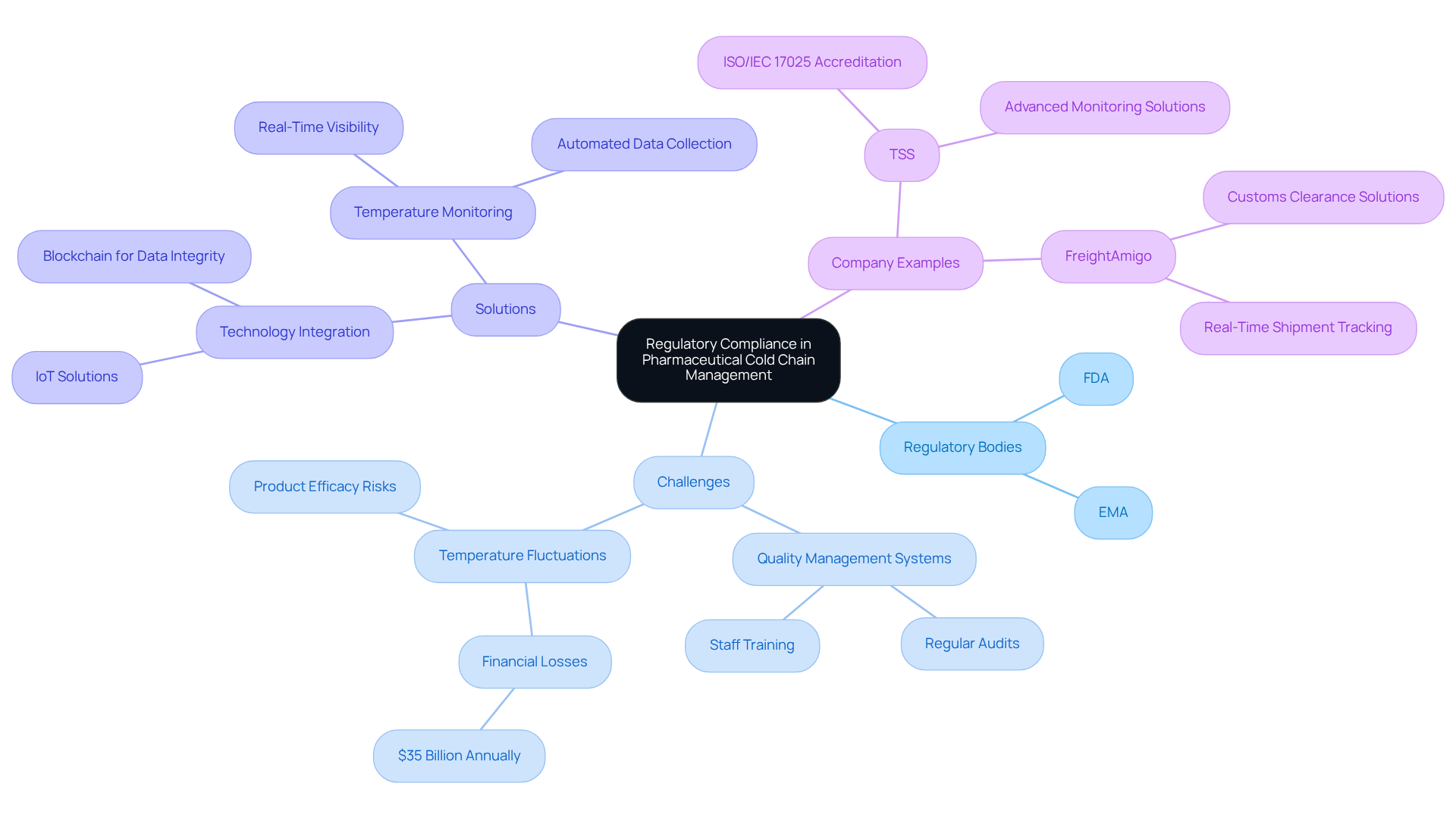
Regulatory Compliance: Navigating Challenges in Pharmaceutical Cold Chain Management
Navigating regulatory compliance in pharmaceutical cold chain management necessitates strict adherence to guidelines established by authorities such as the FDA and EMA. These regulations underscore the importance of climate control, documentation, and traceability throughout the supply chain. Companies face the challenge of establishing robust quality management systems that ensure compliance, which involves conducting regular audits, providing comprehensive training for staff, and maintaining detailed records of monitoring conditions and product handling.
Recent updates from the FDA and EMA highlight an increasing emphasis on comprehensive climate monitoring and data integrity. Regulatory agencies now mandate that pharmaceutical manufacturers utilize validated temperature-controlled packaging and automated data collection systems to ensure real-time visibility into the pharmaceutical cold chain during shipment conditions. This change is critical, as fluctuations in temperature can lead to severe consequences, including diminished effectiveness and potential financial losses, with an estimated $35 billion lost annually due to such fluctuations.
Several companies are successfully navigating these stringent regulations. For instance, TSS has gained recognition for its advanced temperature monitoring solutions, aiding life sciences companies in maintaining compliance with evolving standards. Notably, TSS's Calibration Laboratory achieved ISO/IEC 17025 accreditation by Swedac, reinforcing the credibility of its offerings. Additionally, organizations like FreightAmigo are streamlining customs clearance processes, facilitating compliance for international shipments. It is significant that $8 billion per year is wasted in biologics due to customs delays, underscoring the financial impact of compliance challenges.
As the pharmaceutical industry continues to evolve, awareness of the latest FDA and EMA regulations is crucial for firms aiming to enhance their pharmaceutical cold chain logistics. The integration of advanced technologies, such as IoT and blockchain, is expected to play a pivotal role in improving compliance and operational efficiency in the temperature-sensitive management environment. Furthermore, as David Webber emphasizes, "To stay compliant, drug manufacturers should consistently choose validated climate-controlled packaging, automated climate data collection, and real-time insight into shipment conditions.
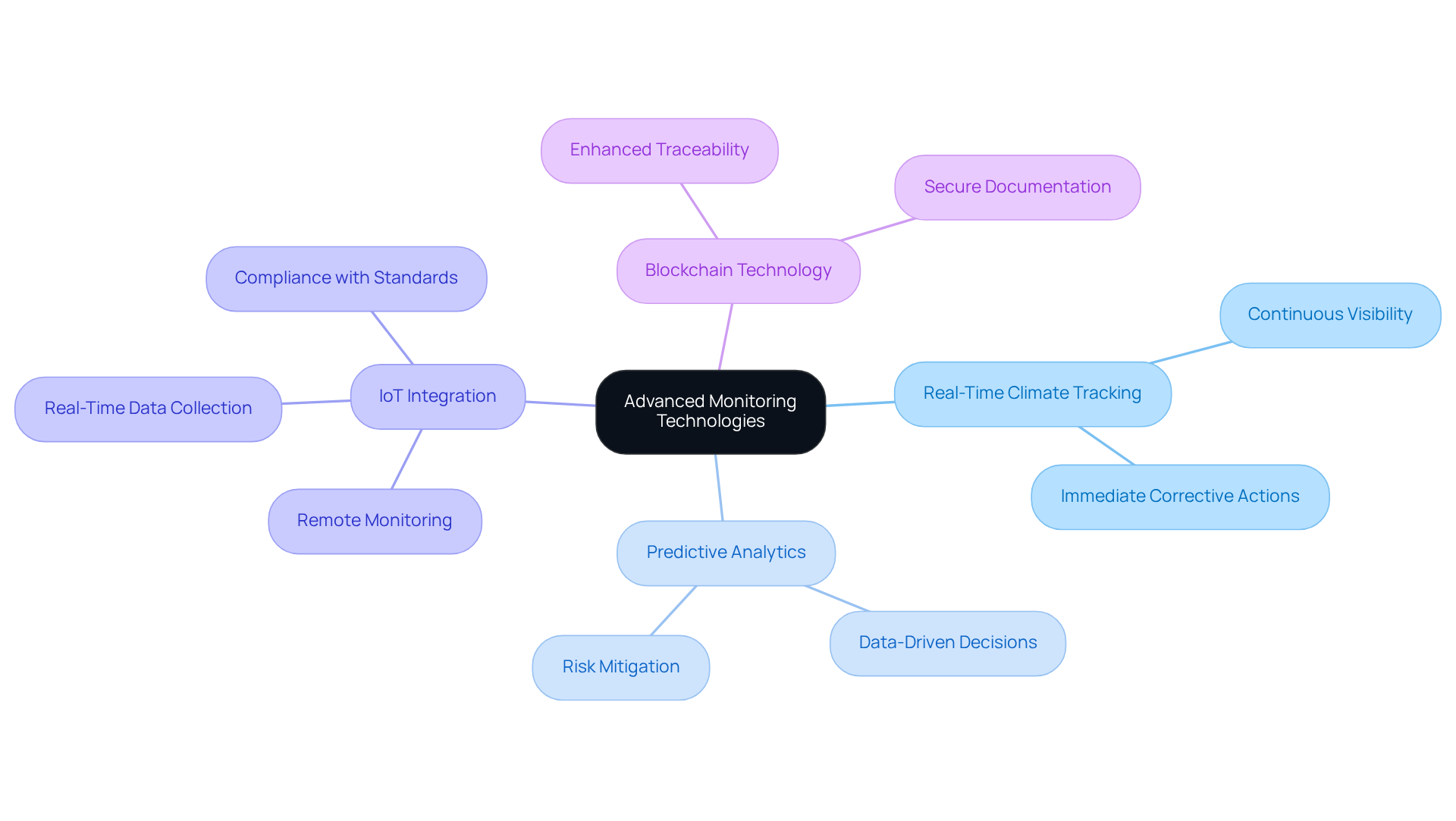
Advanced Monitoring Technologies: Ensuring Cold Chain Effectiveness
Sophisticated monitoring technologies, such as real-time climate tracking systems and predictive analytics, are essential for preserving the integrity of the pharmaceutical cold chain. These systems offer continuous visibility into the conditions of temperature-sensitive products, enabling immediate corrective actions when deviations occur.
For instance, the integration of IoT devices facilitates remote monitoring, providing real-time data on temperature and humidity—critical for compliance with stringent regulatory standards. Furthermore, blockchain technology enhances traceability, ensuring that every stage of the supply network is documented and verifiable.
Statistics indicate that the implementation of IoT solutions in temperature-controlled logistics can significantly reduce spoilage rates, with certain studies demonstrating improvements of up to 30% in product integrity. As the demand for temperature-sensitive drugs continues to escalate, driven by factors such as the global distribution of vaccines and essential medications, the adoption of the pharmaceutical cold chain is increasingly vital for ensuring product safety and regulatory compliance.
Handling Risks: Consequences of Poor Cold Chain Management
Ineffective management of the pharmaceutical cold chain can lead to severe repercussions, including product spoilage, significant financial losses, and reputational damage. A single temperature deviation can render an entire batch of medications unusable, resulting in costly recalls and potential legal liabilities. The drug industry incurs approximately $35 billion annually due to failures in temperature-controlled logistics, underscoring the financial stakes involved.
Moreover, failure to adhere to regulatory standards may result in fines and loss of market access; indeed, 25% of vaccines are damaged due to temperature control issues. As industry experts highlight, the consequences of temperature excursions within the pharmaceutical cold chain extend beyond immediate financial impacts; they can jeopardize patient safety and trust in pharmaceutical products.
Jason Chou, System Vice President of Pharmacy Operations, emphasizes that 'temperature control failures can have harmful impacts for patients, including treatment delays and less than ideal outcomes.' Therefore, it is crucial for companies to implement comprehensive risk management strategies that proactively identify and mitigate these threats, ensuring the integrity of their operations within the pharmaceutical cold chain.
Efficient temperature-controlled risk management relies on four essential pillars:
- Contingency planning
- Robust standard operating procedures
- Digital transformation
- Leveraging technology for proactive risk management
Furthermore, the ecological implications of temperature-controlled logistics failures cannot be overlooked, as product loss significantly increases greenhouse gas emissions due to the necessity for replacement shipments. By addressing these multifaceted challenges, companies can enhance their operational efficiency and sustainability.

Best Practices: Optimizing Pharmaceutical Cold Chain Logistics
To optimize the pharmaceutical cold chain logistics, companies must adopt several best practices that ensure both reliability and efficiency.
- Employ Suitable Packaging Materials: It is essential to select packaging that provides thermal insulation and maintains the required temperature range.
- Implement Real-Time Monitoring: Advanced monitoring systems are crucial for continuously tracking temperature and humidity levels, safeguarding product integrity.
- Conduct Regular Training: All staff involved in the temperature-sensitive processes should receive comprehensive education on best practices and compliance standards to enhance operational effectiveness.
- Establish Contingency Plans: Developing and regularly revising contingency strategies is vital for managing potential interruptions in the temperature-controlled process.
By adhering to these practices, companies can significantly improve their logistics for the pharmaceutical cold chain and ensure the safe delivery of products.
Emerging Trends: Shaping the Future of Pharmaceutical Cold Chain Logistics
The pharmaceutical temperature-controlled logistics industry is undergoing transformative trends, particularly through the implementation of automation and robotics. These technologies significantly streamline operations, minimizing human error and enhancing overall efficiency. For instance, automated temperature control systems can reduce energy usage by 21-26%, aligning with the industry's push for sustainability. Companies are increasingly prioritizing eco-friendly packaging solutions alongside energy-efficient refrigeration systems, reflecting a broader commitment to environmental responsibility. Notably, 50% of worldwide consumers indicate that environmental elements affect their confidence in a brand by 2025, underscoring the significance of sustainability in temperature-controlled logistics.
Moreover, the integration of artificial intelligence and machine learning for predictive analytics is gaining traction. This advancement empowers companies to anticipate potential disruptions, thereby optimizing logistics strategies and improving responsiveness. Consequently, organizations are better prepared to navigate the intricacies of the pharmaceutical cold chain in temperature-controlled logistics, ensuring the integrity of temperature-sensitive pharmaceuticals while fulfilling regulatory compliance and consumer expectations. Statistics reveal that 74% of supply network executives are increasing investments in automation, IoT, and AI technologies by 2025, emphasizing the rising trend toward automation in the sector.
The impact of automation and robotics on temperature-controlled logistics efficiency is profound, with forecasts indicating a potential decrease in transportation expenses by as much as 40% and productivity enhancements ranging from 25% to 70%. This anticipated reduction in logistics costs aligns with the imperative for businesses to streamline processes and enhance efficiency. Furthermore, supply disruptions typically inflate operating costs by 3-5% and decrease sales by about 7%, highlighting the need for automation to mitigate these challenges. These advancements underscore the essential significance of adopting technological innovations to improve operational efficiency within the pharmaceutical cold chain.
Supply Chain Visibility: Key to Effective Cold Chain Management
Supply network transparency stands as a critical pillar of effective temperature-controlled management. By harnessing technologies such as IoT sensors and cloud-based platforms, companies gain real-time insights into the status of their shipments. This enhanced visibility not only facilitates proactive decision-making—allowing companies to tackle potential issues before they escalate—but also supports compliance with regulatory requirements.
It provides a clear audit trail of temperature monitoring and product handling, reinforcing the reliability that stakeholders demand. In today’s competitive landscape, investing in such transparency is not merely beneficial; it is essential for maintaining operational excellence.
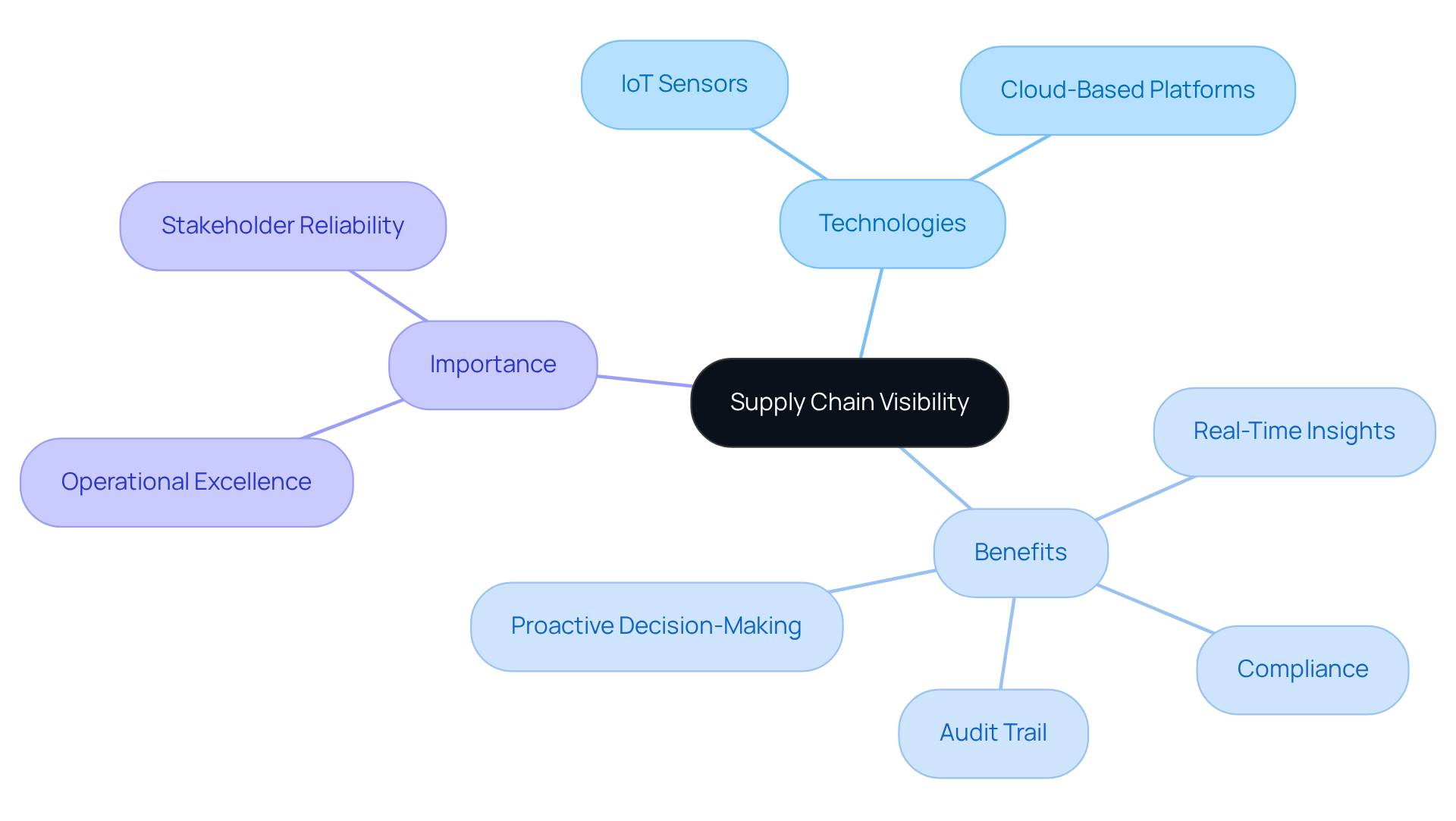
Sustainability: Integrating Eco-Friendly Practices in Cold Chain Logistics
Sustainability in temperature-controlled logistics is increasingly essential, as firms adopt eco-friendly methods that significantly reduce environmental impacts. A key strategy involves utilizing biodegradable packaging materials, which not only minimizes waste but also appeals to the 43% of consumers who prioritize environmental considerations in their purchasing decisions. Furthermore, optimizing transportation routes can lead to substantial reductions in carbon emissions. An international logistics service provider exemplifies this, having achieved a 25% reduction in greenhouse gas emissions through advanced routing algorithms. Additionally, adopting energy-saving refrigeration systems enhances operational efficiency, while transitioning to renewable energy sources for temperature-controlled operations underscores the sector's commitment to sustainability. The refrigerated logistics market is projected to reach $647.47 billion by 2028, highlighting the critical need to incorporate sustainable practices to accommodate this growth. By prioritizing these practices, businesses not only contribute to environmental conservation but also enhance their brand reputation, attracting a growing base of environmentally conscious consumers. As DHL emphasizes, "Utilizing biodegradable, recyclable, or reusable packaging materials to reduce waste is critical for the health of our planet and the long-term sustainability of businesses within the global economy.
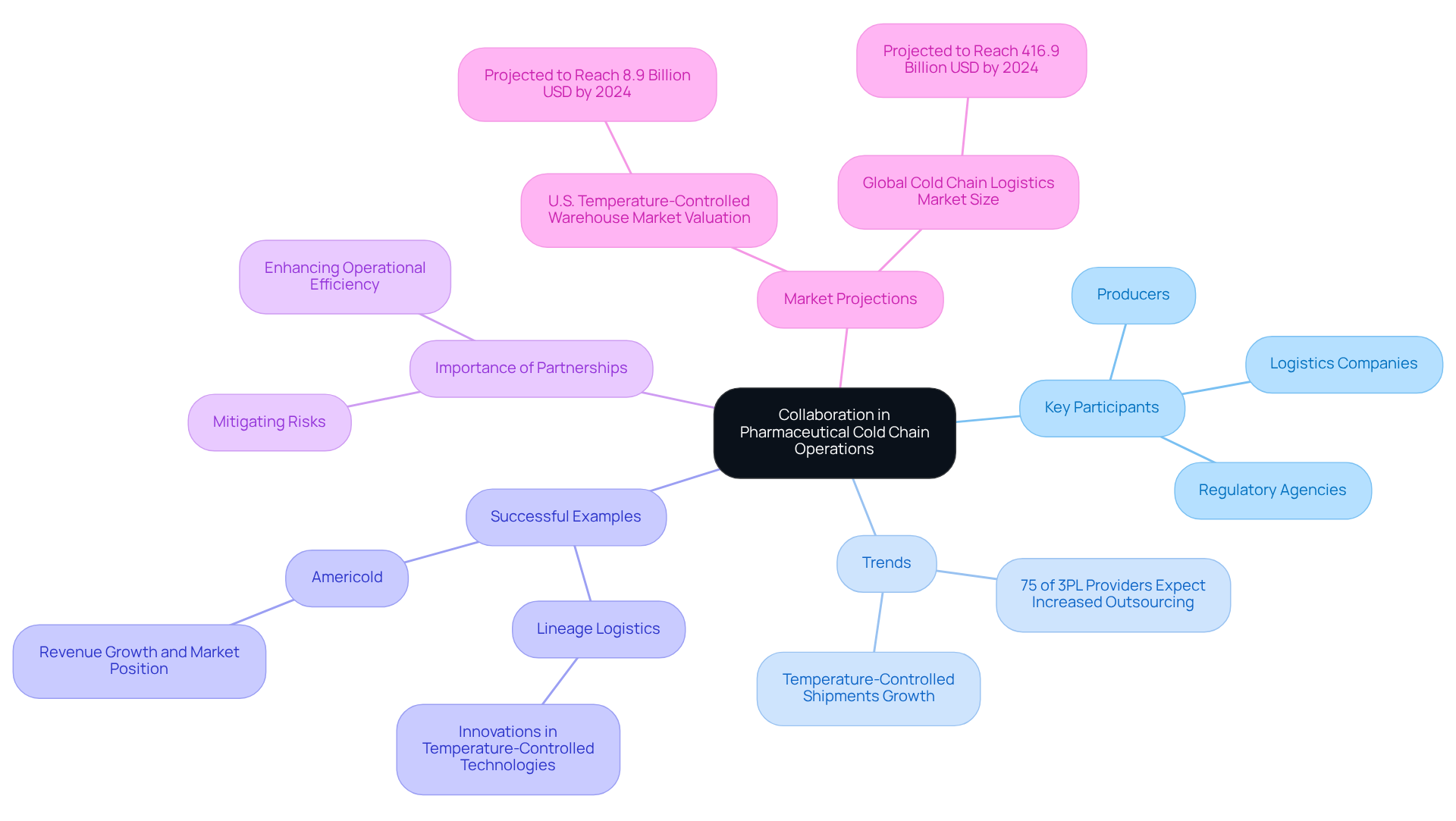
Collaboration: The Key to Successful Pharmaceutical Cold Chain Operations
Efficient drug temperature-controlled operations hinge on robust collaboration among key participants, including producers, logistics companies, and regulatory agencies. By fostering strong partnerships, companies can harness shared resources, knowledge, and best practices, thereby enhancing operational efficiency and mitigating risks. Collaborative strategies not only streamline processes but also ensure compliance with regulatory standards, as stakeholders collectively oversee every facet of the temperature-controlled supply chain.
Recent trends reveal an increasing dependence on partnerships within the drug industry. Notably, 75% of third-party logistics (3PL) providers anticipate a rising demand for outsourcing temperature-sensitive logistics over the next three years, signaling a shift towards specialized expertise. This trend underscores the critical role of collaboration in navigating the complexities of managing temperature-sensitive products. Moreover, temperature-controlled shipments have consistently expanded at a rate twice that of the overall healthcare sector, highlighting the escalating need for efficient temperature-sensitive logistics.
Successful examples of stakeholder collaborations can be seen in the advancements made by companies such as Lineage Logistics and Americold, which have enhanced temperature-controlled technologies essential for the ultra-low conditions required for biologics and gene therapies. Their implementation of smart software and warehouse robotics illustrates how collaborative efforts can yield innovative solutions that preserve the integrity of temperature-sensitive products. The growth and potential of medical temperature-controlled logistics are driven by new treatments and biosimilars, further emphasizing the necessity for cooperation.
Industry leaders stress the importance of these partnerships. As Steve Jobs famously stated, "Great things in business are never done by one person; they’re done by a team of people." This sentiment resonates throughout the healthcare landscape, where collaboration is indispensable for achieving shared goals and ensuring the safety and efficacy of products. Additionally, GPS tracking devices provide real-time location data for precise shipment monitoring, thereby enhancing collaborative efforts in temperature-sensitive logistics.
In conclusion, promoting stakeholder cooperation is not merely beneficial but essential for improving pharmaceutical temperature-controlled logistics. As the industry evolves, the capacity to adapt and innovate through partnerships will be a key driver of success. The U.S. temperature-controlled warehouse market, particularly in the pharmaceutical cold chain sector, is projected to reach a valuation of 8.9 billion USD by 2024, underscoring the significance of effective cold chain management in addressing the growing demands of the market.
Conclusion
The optimization of pharmaceutical cold chain management is crucial for ensuring the safety and efficacy of temperature-sensitive products. By implementing integrated strategies that encompass advanced monitoring technologies, regulatory compliance, and sustainable practices, companies can significantly enhance their operational efficiency. This article emphasizes the need for a comprehensive approach that combines effective packaging, real-time temperature control, and robust collaboration among stakeholders to navigate the complexities of the pharmaceutical supply chain.
Key insights revealed include the importance of precise temperature regulation, which is vital for maintaining product integrity throughout the logistics process. Furthermore, the integration of innovative technologies such as IoT and blockchain enhances supply chain visibility, enabling proactive responses to potential disruptions. The financial and reputational risks associated with poor cold chain management underscore the necessity for businesses to adopt best practices and invest in advanced solutions.
As the pharmaceutical industry evolves, the commitment to optimizing cold chain logistics will be paramount. Companies must prioritize collaboration, embrace emerging technologies, and adopt sustainable practices to meet the growing demands of the market. By doing so, they not only ensure compliance with regulatory standards but also contribute to a safer and more efficient healthcare system. The future of pharmaceutical cold chain management hinges on these strategic initiatives, making it essential for industry players to remain vigilant and adaptable in an ever-changing landscape.
Frequently Asked Questions
What integrated solutions does Western Packaging provide for the pharmaceutical cold chain?
Western Packaging offers a comprehensive strategy combining expert packaging design, filling services, and third-party logistics (3PL) including warehousing, inventory management, and distribution to ensure temperature-sensitive products are handled with care.
Why is temperature control important in the pharmaceutical cold chain?
Temperature control is crucial because even minor fluctuations can compromise the safety and effectiveness of medications. Many pharmaceuticals must maintain a stable temperature range between 2°C and 8°C.
What technologies are used to monitor temperature in the pharmaceutical cold chain?
Advanced thermal monitoring systems, such as IoT sensors and data loggers, are utilized to ensure products remain within critical temperature thresholds during storage and transportation.
What are the benefits of adopting integrated temperature management solutions?
Integrated temperature management solutions improve temperature regulation, enhance operational efficiency, and ensure compliance with regulatory standards, thereby preserving product integrity.
What challenges do companies face regarding regulatory compliance in pharmaceutical cold chain management?
Companies must adhere to strict guidelines from authorities like the FDA and EMA, which require robust quality management systems, regular audits, comprehensive staff training, and detailed monitoring records.
How are recent regulatory updates affecting pharmaceutical cold chain practices?
Recent updates emphasize the need for validated temperature-controlled packaging and automated data collection systems to ensure real-time visibility of conditions during shipment, addressing the risks associated with temperature fluctuations.
What impact do compliance challenges have on the pharmaceutical industry?
Non-compliance can lead to significant financial losses; for instance, an estimated $35 billion is lost annually due to temperature fluctuations, and $8 billion is wasted in biologics due to customs delays.
How can companies enhance their pharmaceutical cold chain logistics?
Companies can enhance their logistics by integrating advanced technologies like IoT and blockchain, utilizing validated climate-controlled packaging, and ensuring real-time monitoring of shipment conditions.




