Overview
The key comparisons between ampules and vials for nutraceutical packaging are critical to understanding their design, use cases, and regulatory compliance.
- Ampules are recognized for providing superior protection and sterility for single doses, making them an ideal choice for products requiring utmost safety.
- In contrast, vials offer versatility, accommodating multiple doses, which can be advantageous for various applications.
This distinction influences selection based on product stability, intended use, and supply chain considerations. Ultimately, the choice between ampules and vials hinges on specific product needs and market demands.
Introduction
The choice between ampules and vials stands as a pivotal decision in the nutraceutical packaging landscape, where safety, efficacy, and consumer trust are paramount. Each packaging type presents distinct advantages and challenges, influencing everything from product integrity to supply chain efficiency. As the nutraceutical market evolves, understanding the nuances of these containers becomes crucial—especially in light of stringent regulatory standards and the growing demand for sustainable practices.
Manufacturers must consider various factors when deciding between these two options. Furthermore, navigating the complexities of compliance and logistics is essential to ensure optimal product delivery.
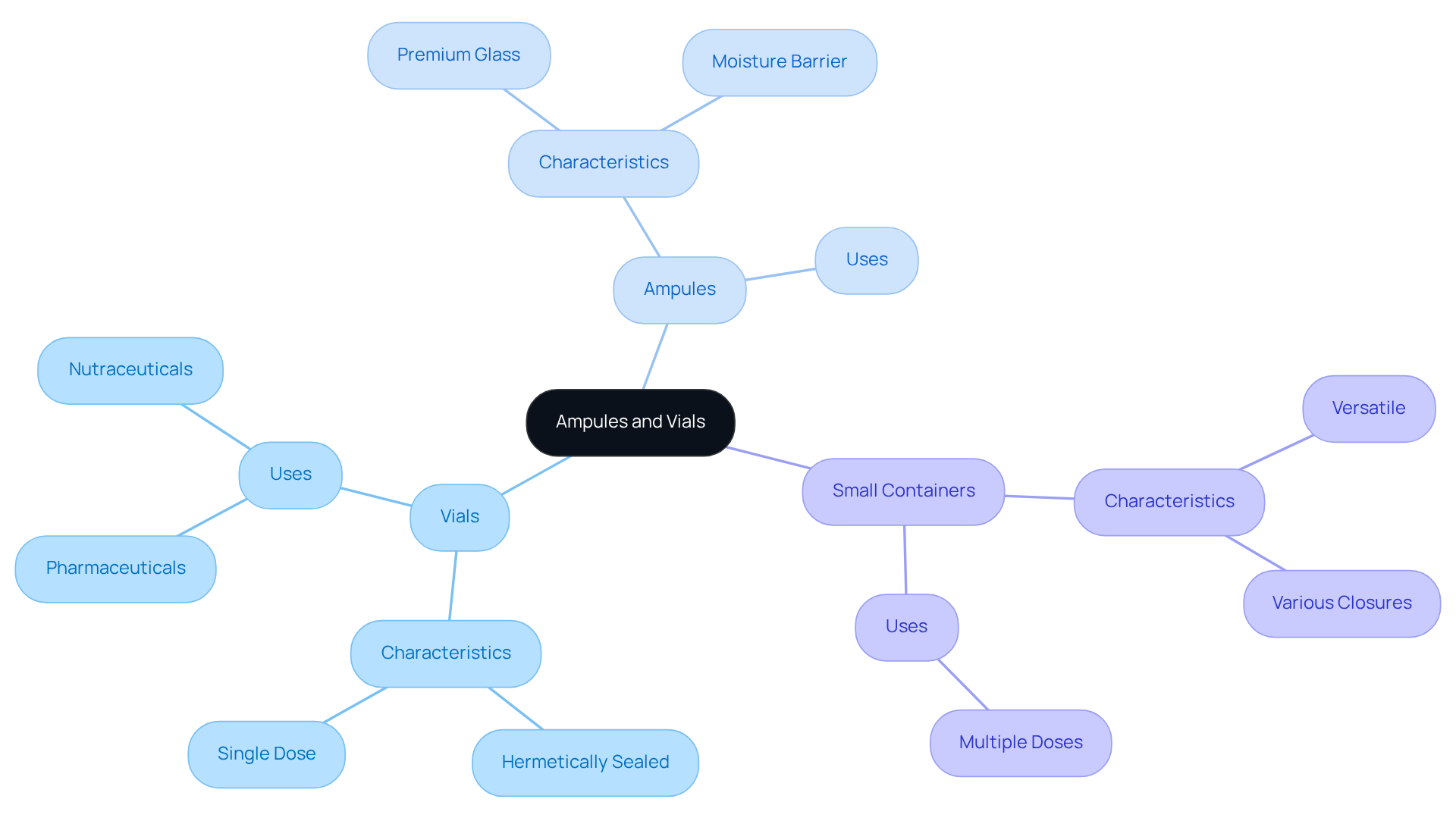
Define Ampules and Vials: Key Characteristics and Uses
Vials are hermetically sealed containers, typically crafted from glass or plastic, designed to hold a single dose of liquid. Their sealed nature protects sensitive formulations from contamination and degradation, making them particularly suitable for pharmaceuticals and nutraceuticals. Ampules, frequently crafted from premium glass, offer outstanding barrier characteristics against moisture and oxygen, ensuring stability. In contrast, small containers are versatile, capable of holding liquids or powders, often intended for multiple doses. They feature various closure options, such as rubber stoppers and screw caps, facilitating easier access and reusability, making them ideal for both consumer and professional applications.
The preference for vials arises from their tamper-resistant design and single-use convenience, ensuring item integrity. Conversely, containers are favored for their versatility and ability to accommodate greater amounts, serving various demands in the nutraceutical industry. As market dynamics evolve, the demand for both packaging types, specifically ampule vs vial, is anticipated to increase, with each playing essential roles in providing safe and effective nutraceutical offerings. At Western Packaging, we provide integrated filling services that enhance our customized flexible packaging solutions, boosting appeal and fulfilling distinct needs. Our innovative packaging design solutions elevate brand recognition and shelf appeal, ensuring your products stand out in a competitive market.
According to market projections, the North America Pharmaceutical Glass Packaging Market is expected to reach USD 7.96 billion by 2030, driven by increasing consumer spending on healthcare and the demand for glass packaging solutions. This trend underscores the importance of both in meeting the evolving needs of the nutraceutical industry.
Compare Advantages and Disadvantages of Ampules and Vials
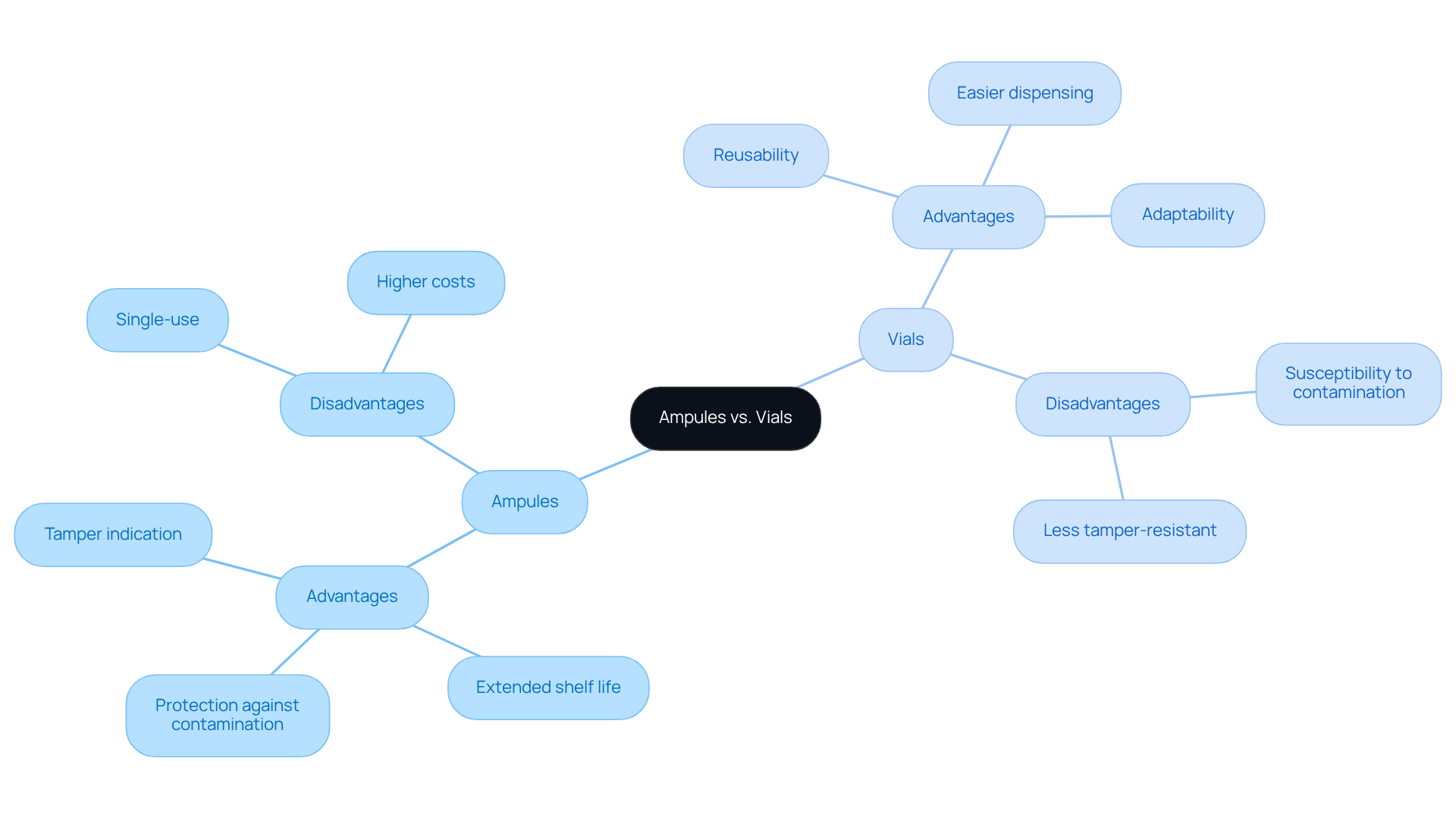
Ampules present distinct advantages, including:
- Superior protection against contamination
- An extended shelf life due to their sealed nature
- A clear indication of tampering
However, their single-use characteristic can lead to increased waste and higher costs per dose. Conversely, vials offer greater adaptability and can be reused, making them an economical choice for items that require multiple doses. They facilitate easier dispensing and can accommodate a broader range of formulations. Nonetheless, vials may be more susceptible to contamination if not properly sealed, and their closures can sometimes be less tamper-resistant compared to other types. Ultimately, the decision between ampule vs vial hinges on the item's stability, intended use, and market positioning.
Examine Regulatory Compliance and Quality Control Issues
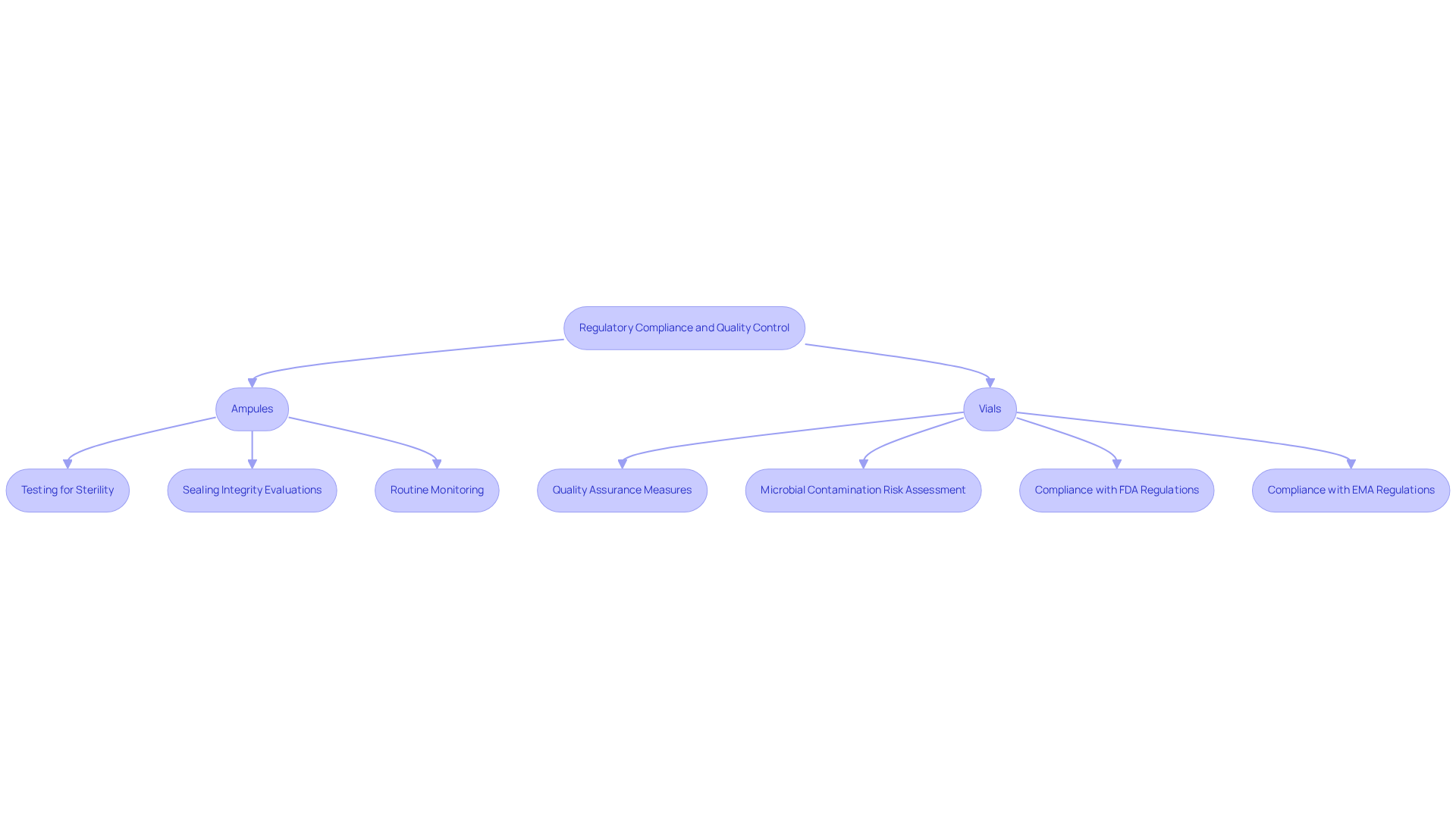
Containers and bottles are governed by stringent regulatory standards established by organizations such as the FDA and EMA, which are vital for maintaining quality in the nutraceutical sector. When comparing , it is important to note that ampules are specifically designed for sensitive formulations and undergo thorough testing for sterility and stability. Their hermetically sealed design significantly reduces exposure to contaminants, thereby simplifying compliance with safety regulations. Conversely, in the discussion of ampule vs vial, containers require additional quality assurance measures to ensure that seals remain secure and that the product stays uncontaminated post-opening. This encompasses regular evaluations of sealing integrity and the risk of microbial contamination.
Manufacturers must navigate these complexities with precision, as non-compliance can result in costly recalls and severe damage to brand reputation. For instance, the FDA's 2025 regulations underscore the necessity for comprehensive quality control protocols tailored to each type of packaging, including routine sterility testing for small glass containers and stability studies for other formats. Understanding these regulatory requirements is essential for ensuring that nutraceutical products not only meet safety and efficacy standards but also align with consumer expectations for quality.
Moreover, periodic medical examinations for workers handling hazardous drugs should occur every one to three years, underscoring the significance of ongoing quality control and compliance monitoring. Regulatory authorities emphasize the importance of consistent monitoring and adherence to guidelines, with OSHA highlighting that surveillance serves as a formal assessment of worker groups, ensuring the effectiveness of existing controls. Successful quality control practices include regular sterility assessments for containers and stability studies for bottles, which help identify potential issues before products reach the market. Furthermore, insights from case studies on dimensional standards for glass containers and the classification of glass types can enrich the discussion on quality control measures, thus safeguarding public health and maintaining trust in nutraceutical offerings.
Analyze Impact on Supply Chain Efficiency and Logistics
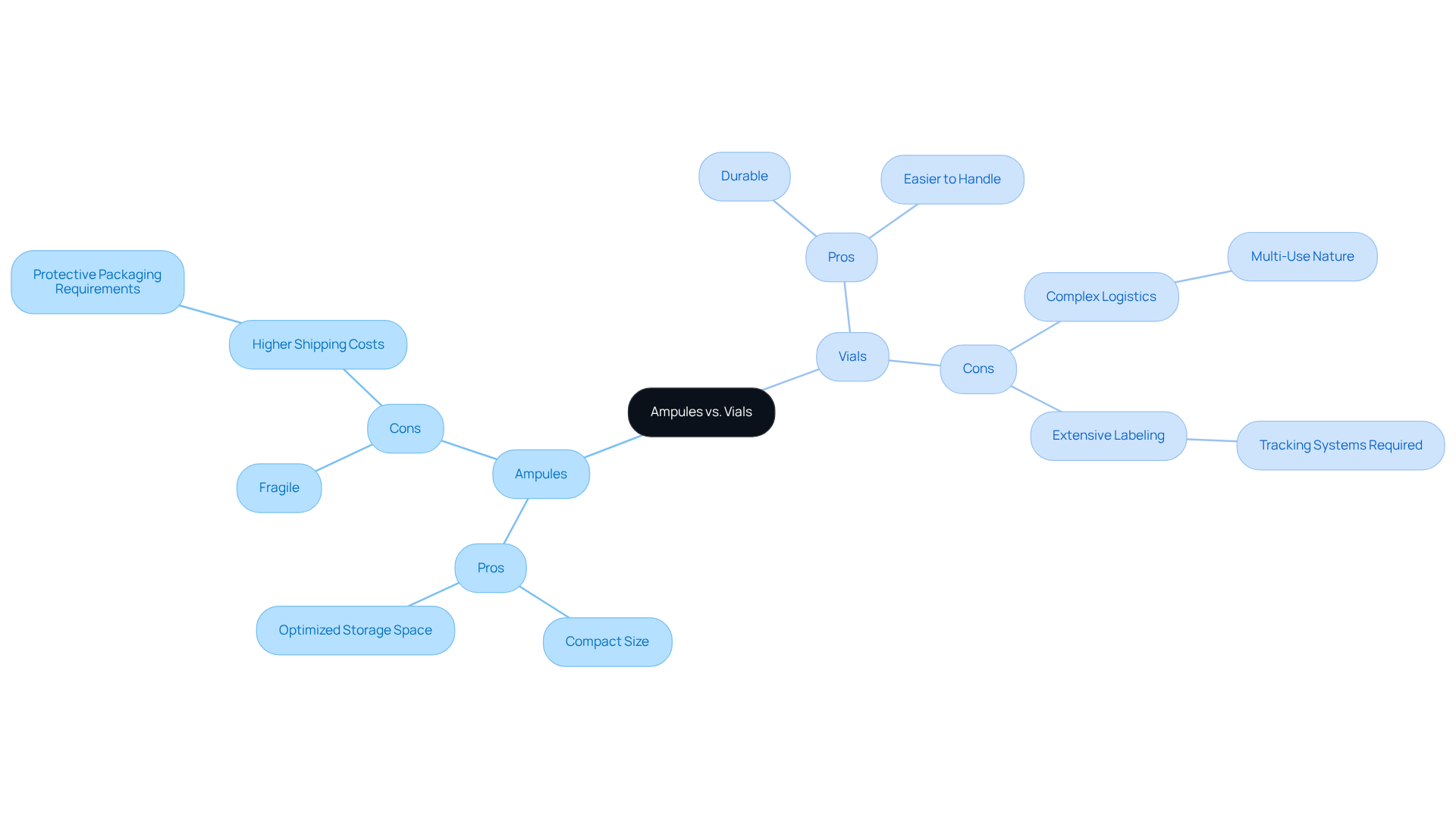
The choice of ampule vs vial is critical for optimizing supply chain efficiency. Vials, designed for one-time use, require careful handling due to their fragile nature, which can lead to stemming from the need for protective packaging. However, their compact design often results in more efficient storage space utilization. Conversely, containers are typically more durable and easier to handle; yet, their multi-use capability introduces logistical challenges, particularly in stock control. This complexity is further exacerbated by the need for comprehensive labeling and tracking systems to ensure accurate dispensing.
Pros and Cons of Ampules vs. Vials:
- Ampules:
- Pros: Compact size, optimized storage space.
- Cons: Fragile, higher shipping costs due to protective packaging requirements.
- Vials:
- Pros: Durable, easier to handle.
- Cons: Complex logistics due to multi-use nature, extensive labeling and tracking systems required.
Consequently, the decision regarding ampule vs vial must consider not only the unique characteristics of the product but also the broader implications for supply chain management and operational efficiency. Addressing these logistical challenges is vital for manufacturers and distributors in the nutraceutical industry to enhance overall effectiveness. Furthermore, with 94% of consumers more likely to remain loyal to brands that prioritize transparency, the significance of clear labeling cannot be overstated in this context.
Conclusion
The comparison between ampules and vials underscores the distinct roles each packaging type plays within the nutraceutical industry. Ampules, offering superior protection and stability for sensitive formulations, stand in contrast to vials, which provide versatility and the option for multiple doses. Understanding these differences is essential for manufacturers aiming to select the most effective packaging solution that aligns with their product requirements and market strategies.
Key insights reveal that ampules excel in preventing contamination and ensuring product integrity; however, their single-use nature can lead to higher costs and waste. In contrast, vials are more adaptable and economical for multi-dose applications, yet they require stringent quality control measures to maintain safety and efficacy. The implications of these packaging decisions extend beyond product safety, impacting supply chain efficiency and logistics, as each type presents unique challenges and benefits.
Ultimately, the choice between ampules and vials should be informed by a thorough evaluation of product stability, regulatory compliance, and logistical efficiency. As the nutraceutical market continues to evolve, prioritizing the right packaging enhances product appeal and ensures adherence to safety standards and consumer expectations. Embracing innovative packaging solutions is crucial for a brand's success in a competitive landscape, making it essential for stakeholders to remain informed and proactive in their packaging strategies.
Frequently Asked Questions
What are vials and their key characteristics?
Vials are hermetically sealed containers made from glass or plastic, designed to hold a single dose of liquid. Their sealed nature protects sensitive formulations from contamination and degradation, making them suitable for pharmaceuticals and nutraceuticals.
How do ampules differ from vials?
Ampules are typically crafted from premium glass and offer excellent barrier characteristics against moisture and oxygen, ensuring stability. While vials are designed for single use, ampules are also sealed to protect their contents.
What are small containers used for?
Small containers are versatile and can hold liquids or powders, often intended for multiple doses. They come with various closure options, such as rubber stoppers and screw caps, making them ideal for both consumer and professional applications.
Why are vials preferred in certain applications?
Vials are favored for their tamper-resistant design and single-use convenience, which ensures the integrity of the contents.
What advantages do small containers offer?
Small containers are valued for their versatility and ability to accommodate greater amounts of product, catering to various demands in the nutraceutical industry.
What is the market outlook for pharmaceutical glass packaging?
The North America Pharmaceutical Glass Packaging Market is projected to reach USD 7.96 billion by 2030, driven by increasing consumer spending on healthcare and the demand for glass packaging solutions.
What role do ampules and vials play in the nutraceutical industry?
Both ampules and vials are essential for providing safe and effective nutraceutical offerings, with each serving distinct needs as market dynamics evolve.




