Overview
This article delves into the critical components essential for achieving success in the nutraceutical industry, with a particular focus on assembly and packaging processes. It underscores the necessity of adhering to regulatory standards, implementing robust quality control measures, optimizing supply chain management, and leveraging integrated packaging solutions. These elements are vital for maintaining product quality and meeting consumer expectations in a competitive market. By prioritizing these strategies, industry professionals can enhance their operational efficiency and ensure their products stand out in a crowded marketplace.
Introduction
Navigating the intricate landscape of the nutraceutical industry demands a keen understanding of the evolving standards and regulations that govern product safety and consumer trust. As the sector anticipates significant growth, companies are presented with a unique opportunity to enhance their operational efficiency and brand credibility through the implementation of robust quality control measures and strategic supply chain management.
However, with the impending regulatory changes set to impact labeling and claims verification in 2025, businesses must adapt effectively to these challenges.
How can they ensure their products stand out in a competitive market? This question underscores the urgency for companies to reassess their strategies and embrace innovative solutions that not only comply with regulatory demands but also elevate their market presence.
Understand Nutraceutical Industry Standards and Regulations
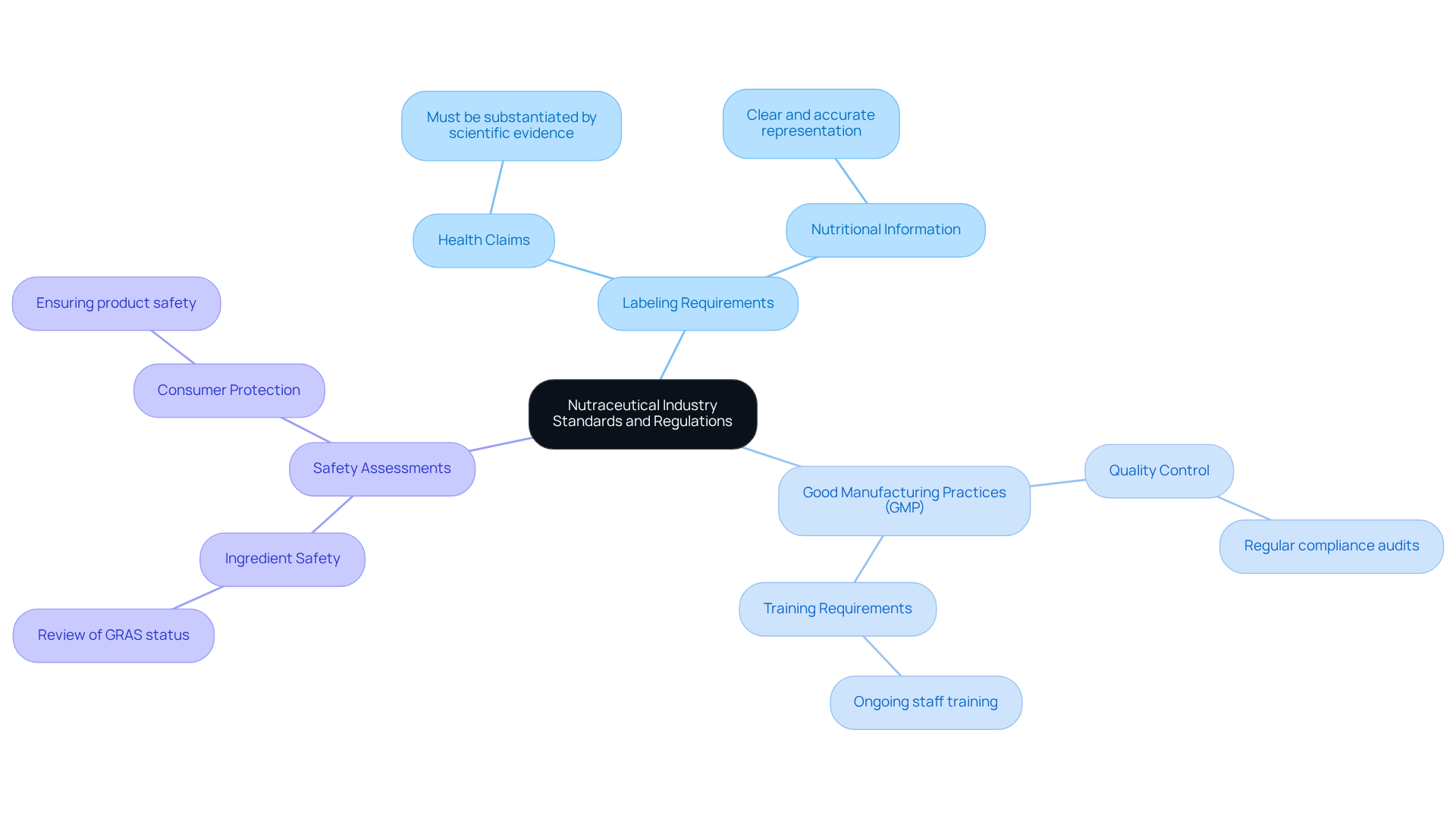
The dietary supplement sector operates within a complex regulatory framework that varies by region and product type. In the United States, the FDA governs dietary supplements through the Dietary Supplement Health and Education Act (DSHEA), which establishes essential guidelines, including:
- Labeling requirements
- Good Manufacturing Practices (GMP)
- Safety assessments
Notably, the FDA mandates that all health claims on labels be substantiated by scientific evidence, ensuring consumers receive accurate information about the products they use. As the industry progresses, companies must stay alert to regulatory updates, such as the FDA's forthcoming rules on labeling accuracy and claims verification, set to take effect in 2025.
Regular training and compliance audits are crucial for maintaining adherence to these standards, which not only protect consumer safety but also enhance brand credibility. With the dietary supplement market anticipated to grow significantly, understanding and complying with these regulations is vital for achieving success.
Implement Robust Quality Control Measures
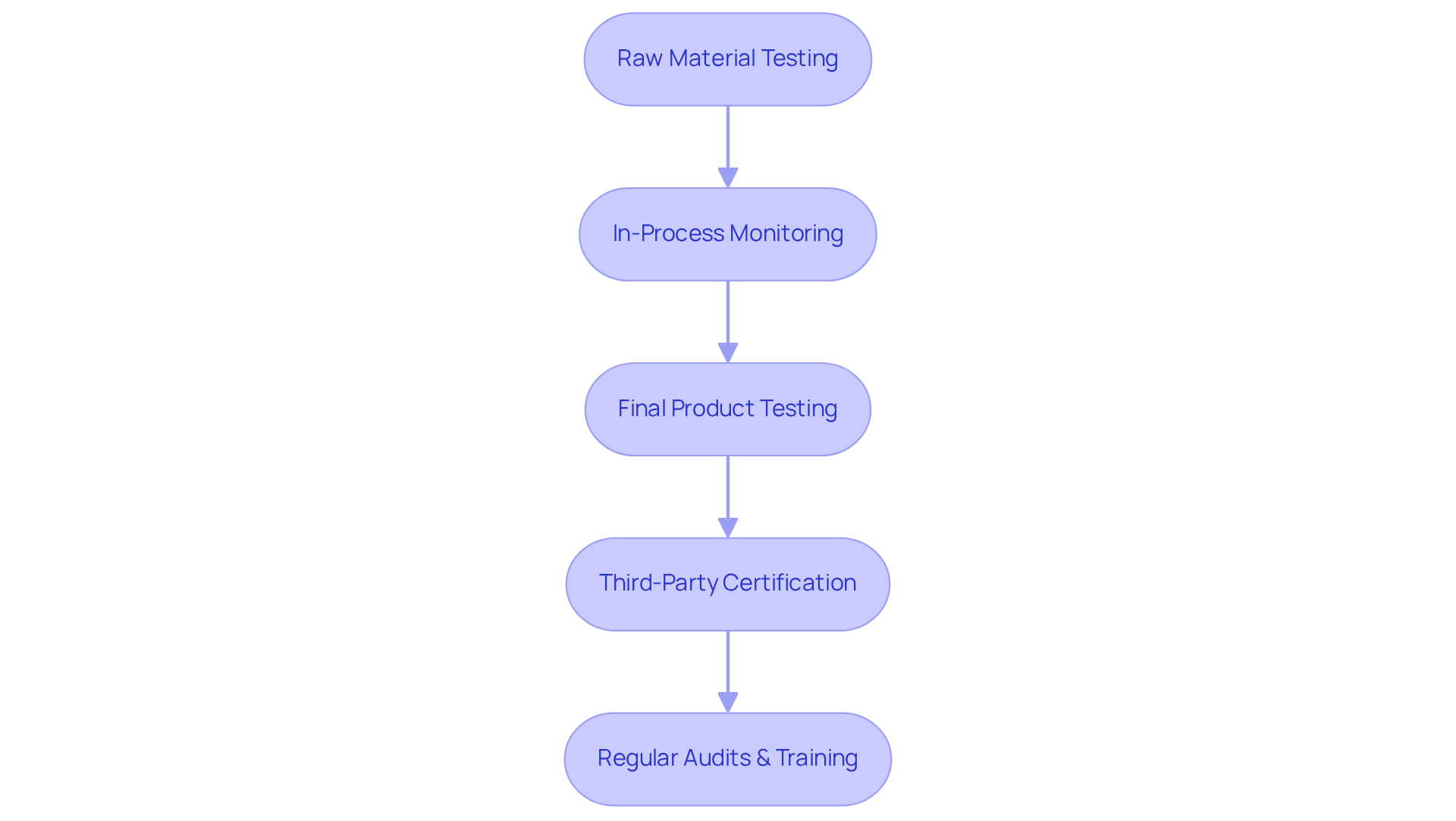
To uphold exceptional standards in the nutraceutical sector, companies must implement comprehensive quality control protocols that encompass every stage of production. This necessitates rigorous testing of raw materials to identify contaminants and confirm potency, along with in-process monitoring to guarantee adherence to specifications. Final product testing is crucial for verifying label claims.
Engaging third-party testing and certifications, such as the USP Verification Mark, significantly enhances the credibility of the offering. Furthermore, establishing a robust system for maintaining standards, which includes regular audits and staff training on excellence criteria, is vital.
A significant case analysis of a leading nutraceutical brand demonstrated that the implementation of a strong Quality Management System (QMS) led to a 30% reduction in defects and a notable increase in customer satisfaction ratings, underscoring the critical impact of excellence oversight on operational success.
Despite 94% of survey participants having a dedicated assurance function and 97% establishing control processes, recalls persist as a challenge. This highlights the ongoing difficulties in product oversight, particularly as 96% of organizations report experiencing a product recall in the last five years.
The escalating costs associated with quality can account for up to 20% of income for many organizations, further emphasizing the financial implications of effective quality oversight.
Optimize Supply Chain Management for Packaging Efficiency
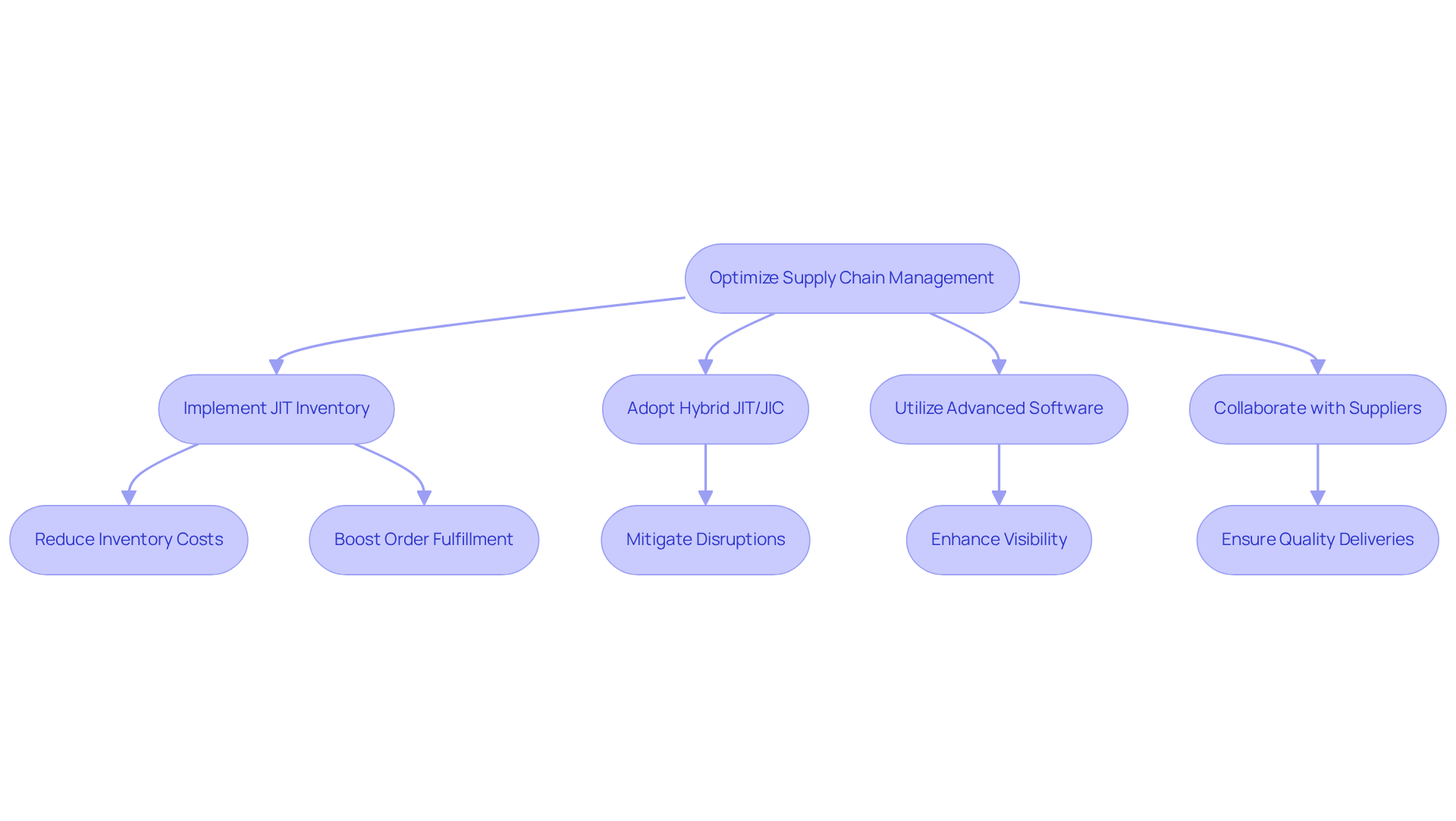
To enhance supply chain oversight, health supplement companies must adopt a comprehensive strategy that seamlessly integrates procurement, production, and distribution. Implementing just-in-time (JIT) inventory systems is crucial for minimizing waste and reducing storage costs. However, in today's supply chain landscape, a hybrid approach that merges JIT with just-in-case (JIC) strategies is increasingly vital to mitigate potential disruptions.
For instance, a nutraceutical manufacturer that implemented a JIT approach realized a 20% reduction in inventory costs while significantly boosting order fulfillment rates. Furthermore, utilizing advanced supply chain management software improves visibility throughout the supply chain, enabling informed decision-making through features such as real-time tracking and predictive analytics.
Collaborating with reliable suppliers and logistics partners is essential for maintaining quality and ensuring timely deliveries. By regularly reviewing and adapting supply chain strategies in response to changing market conditions, companies can further optimize operational efficiency.
Leverage Integrated Packaging Solutions for Enhanced Efficiency
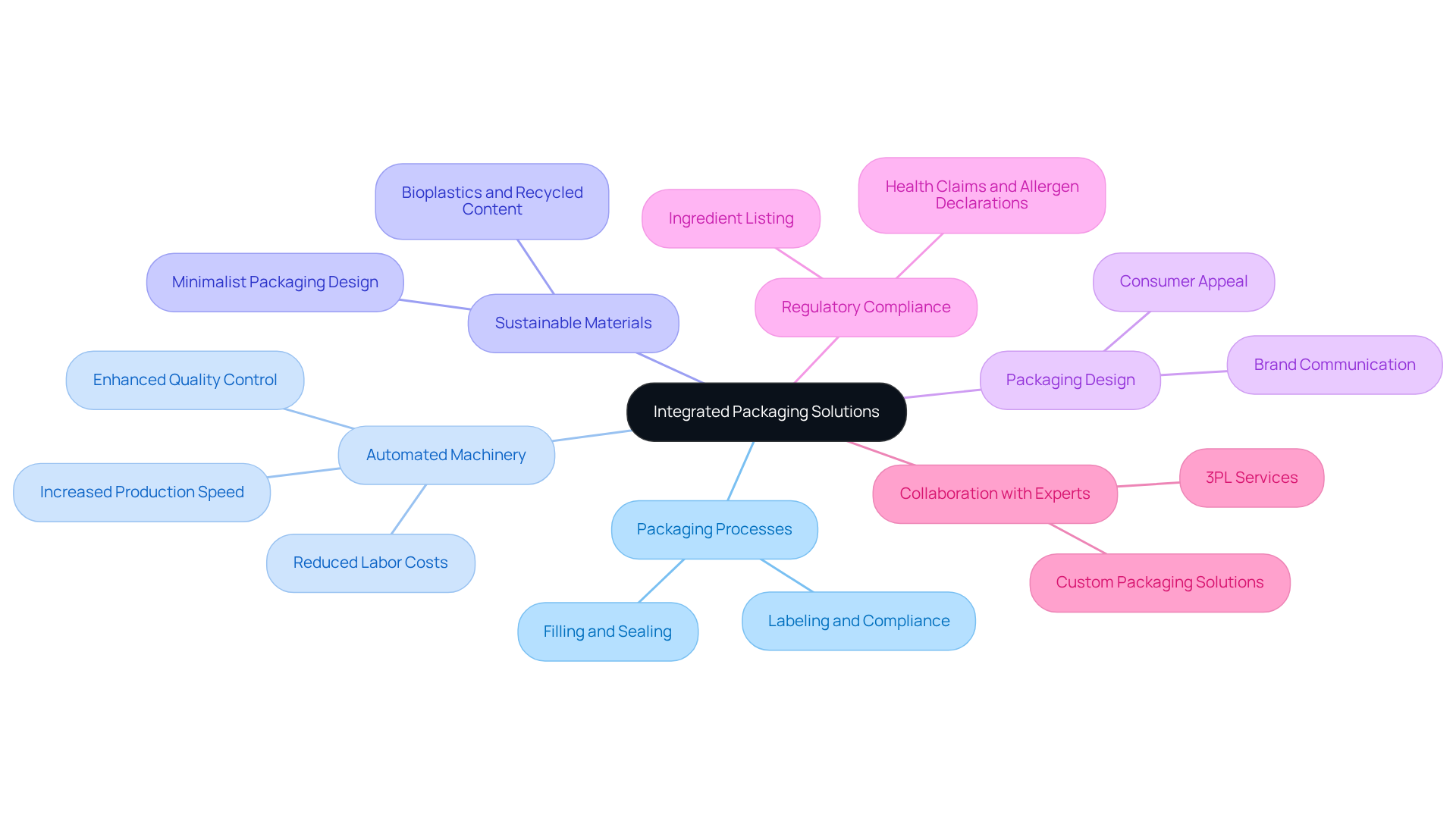
Incorporating packaging solutions is essential for nutraceutical firms aiming to optimize their packaging processes and enhance product appeal. Western Packaging's filling process seamlessly integrates into comprehensive solutions, catering to a diverse range of items from powders to gummies and soft gels.
Automated packaging machinery is pivotal in this transformation, significantly reducing labor costs while increasing production speed. Moreover, the adoption of sustainable packaging materials resonates with environmentally conscious consumers, thereby fostering brand loyalty.
It is imperative for companies to prioritize packaging design, ensuring it not only protects the product but also effectively communicates the brand's values and benefits. Additionally, managing regulatory compliance challenges is crucial, as dietary supplement packaging must adhere to various standards.
By collaborating with packaging experts like Western Packaging, which also provides comprehensive 3PL services, companies can achieve innovative solutions that meet both functional and aesthetic requirements, ultimately driving success in the competitive nutraceutical market.
Conclusion
Understanding the intricacies of the nutraceutical industry is essential for companies aiming to thrive in a competitive market. By focusing on:
- Compliance with industry standards and regulations,
- Implementing robust quality control measures,
- Optimizing supply chain management, and
- Leveraging integrated packaging solutions,
businesses can create a strong foundation for success.
Key insights from the article highlight the importance of adhering to regulatory guidelines set forth by authorities like the FDA. These guidelines not only safeguard consumer health but also enhance brand credibility. Furthermore, implementing rigorous quality control protocols and adopting innovative supply chain strategies can significantly improve product quality and operational efficiency. The integration of advanced packaging solutions further contributes to enhancing product appeal and meeting regulatory requirements.
Ultimately, the path to success in the nutraceutical sector hinges on a proactive approach to compliance, quality assurance, and operational excellence. Companies that prioritize these best practices will navigate the complexities of the industry more effectively and resonate with consumers, ensuring long-term growth and sustainability. Embracing these strategies is crucial for any nutraceutical company striving to make a mark in the evolving marketplace.
Frequently Asked Questions
What governs the dietary supplement sector in the United States?
The dietary supplement sector in the United States is governed by the FDA through the Dietary Supplement Health and Education Act (DSHEA).
What are some key guidelines established by the DSHEA?
Key guidelines established by the DSHEA include labeling requirements, Good Manufacturing Practices (GMP), and safety assessments.
What is required for health claims made on dietary supplement labels?
The FDA mandates that all health claims on dietary supplement labels must be substantiated by scientific evidence to ensure consumers receive accurate information.
What upcoming regulatory changes should companies be aware of?
Companies should be aware of the FDA's forthcoming rules on labeling accuracy and claims verification, which are set to take effect in 2025.
Why are regular training and compliance audits important in the nutraceutical industry?
Regular training and compliance audits are crucial for maintaining adherence to industry standards, which protect consumer safety and enhance brand credibility.
What is the outlook for the dietary supplement market?
The dietary supplement market is anticipated to grow significantly, making understanding and complying with regulations vital for achieving success in the industry.




