Overview
This article delves into best practices for dietary supplement packaging, highlighting the critical nature of:
- Regulatory compliance
- Effective design strategies
- Operational efficiency
- Streamlined distribution
By adhering to FDA guidelines, implementing automation in filling processes, and leveraging third-party logistics, companies can significantly enhance product safety, foster consumer trust, and boost market competitiveness.
These specific actions not only ensure compliance but also position brands favorably within the industry, ultimately driving success. Embracing these best practices is essential for any organization aiming to excel in the competitive landscape of dietary supplements.
Introduction
In an industry where consumer trust hinges on quality and transparency, dietary supplement packaging plays a pivotal role in shaping brand perception and driving sales. By mastering best practices in packaging—from regulatory compliance to innovative design—manufacturers can significantly enhance their market presence and foster consumer loyalty. However, as the landscape evolves and competition intensifies, companies must navigate the complexities of packaging effectively while meeting consumer expectations and regulatory standards.
Understand Quality Standards and Regulatory Compliance
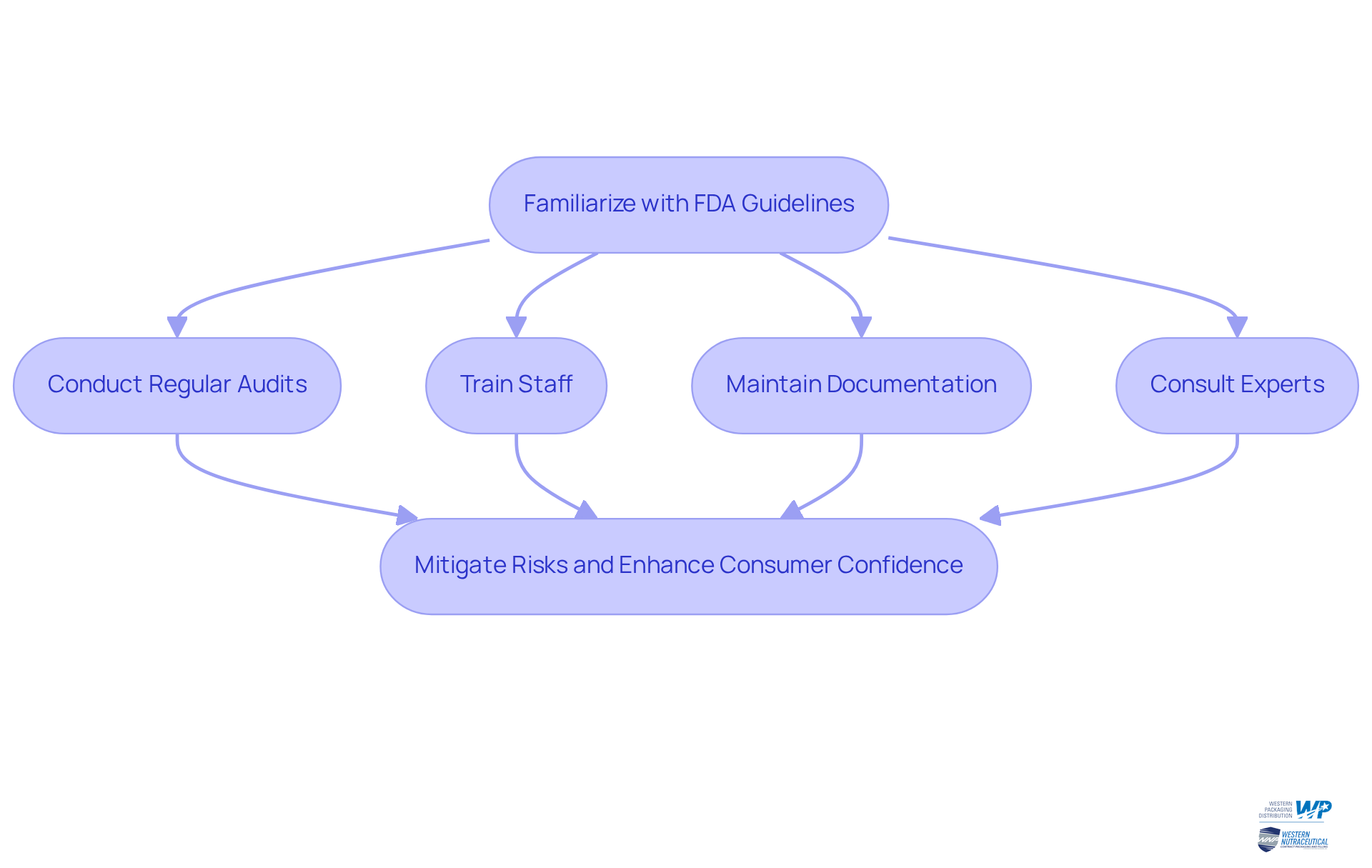
To adeptly navigate the intricate landscape of dietary supplement regulations, manufacturers must familiarize themselves with the FDA's guidelines, particularly the Current Good Manufacturing Practices (cGMPs) detailed in 21 CFR Part 111. This involves ensuring that all dietary supplement packaging materials are safe, suitable, and compliant with labeling requirements. Key steps to achieve this include:
- Conducting Regular Audits: Regularly review container processes and materials to ensure compliance with FDA regulations.
- Training Staff: Ensure that all employees involved in handling products are trained on regulatory requirements and quality standards.
- Documentation: Maintain thorough records of all material and processes to demonstrate compliance during inspections.
- Consulting Experts: Engage with regulatory consultants or legal advisors to stay updated on changes in regulations and best practices.
By adhering to these practices, companies can mitigate risks associated with non-compliance and enhance consumer confidence in their offerings.
Implement Effective Packaging Design Strategies
To create packaging that stands out in a competitive market, consider the following strategies:
-
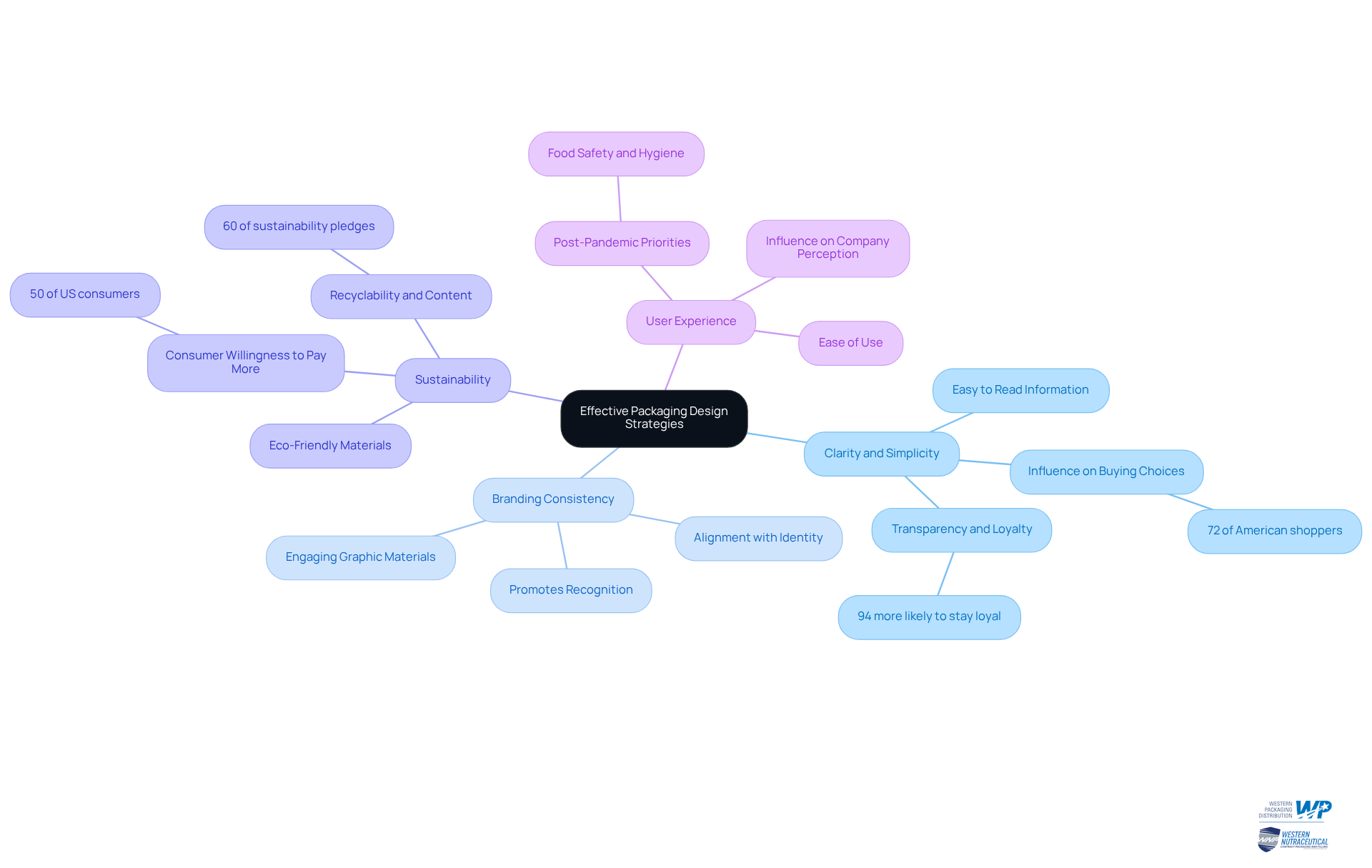
Clarity and Simplicity: Ensure that product information is easy to read and understand. Distinct fonts and tidy designs are crucial, as 72% of American shoppers indicate that design influences their buying choices. Moreover, clear labeling promotes loyalty to companies, with 94% of individuals more likely to stay loyal to organizations that provide transparency.
-
Branding Consistency: Align packaging design with your overall identity, including colors, logos, and messaging. This consistency promotes recognition and loyalty, essential in a market where customers appreciate transparency. Western Packaging focuses on creating engaging graphic materials, ensuring a unified and captivating identity that connects with customers.
-
Sustainability: Incorporate eco-friendly materials and designs, as approximately 50% of US consumers are willing to pay more for sustainable containers. This trend not only boosts customer loyalty but also attracts environmentally aware consumers, with 60% of sustainability pledges emphasizing complete recyclability of materials and greater recycled content.
-
User Experience: Design containers that are easy to open and use, enhancing customer satisfaction and promoting repeat purchases. A seamless user experience can significantly influence company perception and sales, especially as consumers have prioritized food safety and hygiene following the COVID-19 pandemic. Western Packaging provides inventive flexible packaging options customized to your requirements, such as large pouches for protein items and stick packs for nutraceuticals, enhancing appeal and usability.
Real-world instances include companies that have effectively employed minimalist designs to boost product attraction while ensuring adherence to labeling regulations. These strategies not only satisfy customer expectations but also position brands advantageously in the expanding dietary supplement packaging market, projected to exceed $220 billion by 2027. Furthermore, it is essential to steer clear of typical mistakes in design, such as overcomplicating concepts or overlooking regulatory adherence, to ensure effectiveness and consumer trust.
Integrate Filling Services for Operational Efficiency
To enhance the filling process within your packaging operations, consider implementing the following best practices:
-
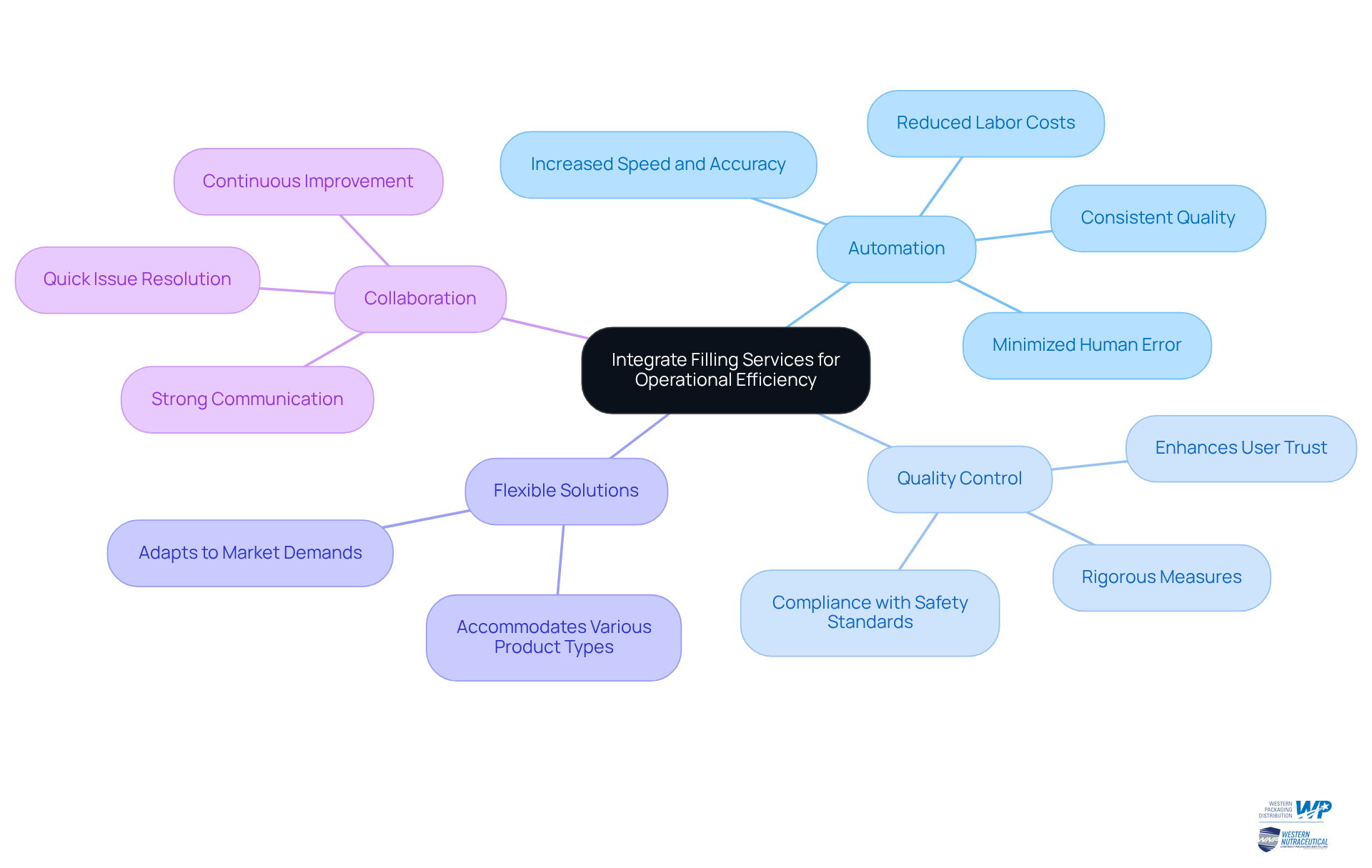
Automation: Investing in automated filling equipment significantly boosts speed and accuracy, leading to reduced labor costs and minimized human error. Automation not only simplifies operations but also guarantees consistent quality, essential in the nutraceutical sector. Notably, over 70% of food industry executives have reported outsourcing some operations to improve efficiency, highlighting the critical role of automation in maintaining a competitive advantage.
-
Quality Control: Establishing rigorous quality control measures during the filling process is essential for maintaining consistency and compliance with safety standards. Industry leaders emphasize that effective quality control in dietary supplement packaging can prevent costly mistakes and enhance trust among users. As W. Edwards Deming stated, "Quality is everyone's responsibility," underscoring the collective effort required to uphold high standards.
-
Flexible Solutions: Opt for filling systems designed to accommodate a variety of product types and sizes. This flexibility allows businesses to adapt swiftly to changing market demands, ensuring they meet consumer preferences without compromising quality. The ability to adjust to market needs is vital in the fast-paced nutraceutical industry.
-
Collaboration: Encourage strong communication between packaging and filling teams. This collaboration is crucial for seamless operations and enables quick resolution of any issues that may arise, ultimately improving overall efficiency. As Taiichi Ohno, the father of the Toyota Production System, emphasized, continuous improvement in procedures is essential for operational success.
By integrating these practices, companies can significantly enhance operational efficiency and improve delivery timelines, positioning themselves for success in the competitive nutraceutical market.
Leverage Third-Party Logistics for Streamlined Distribution
To fully leverage third-party logistics in your distribution strategy, consider these key strategies:
-
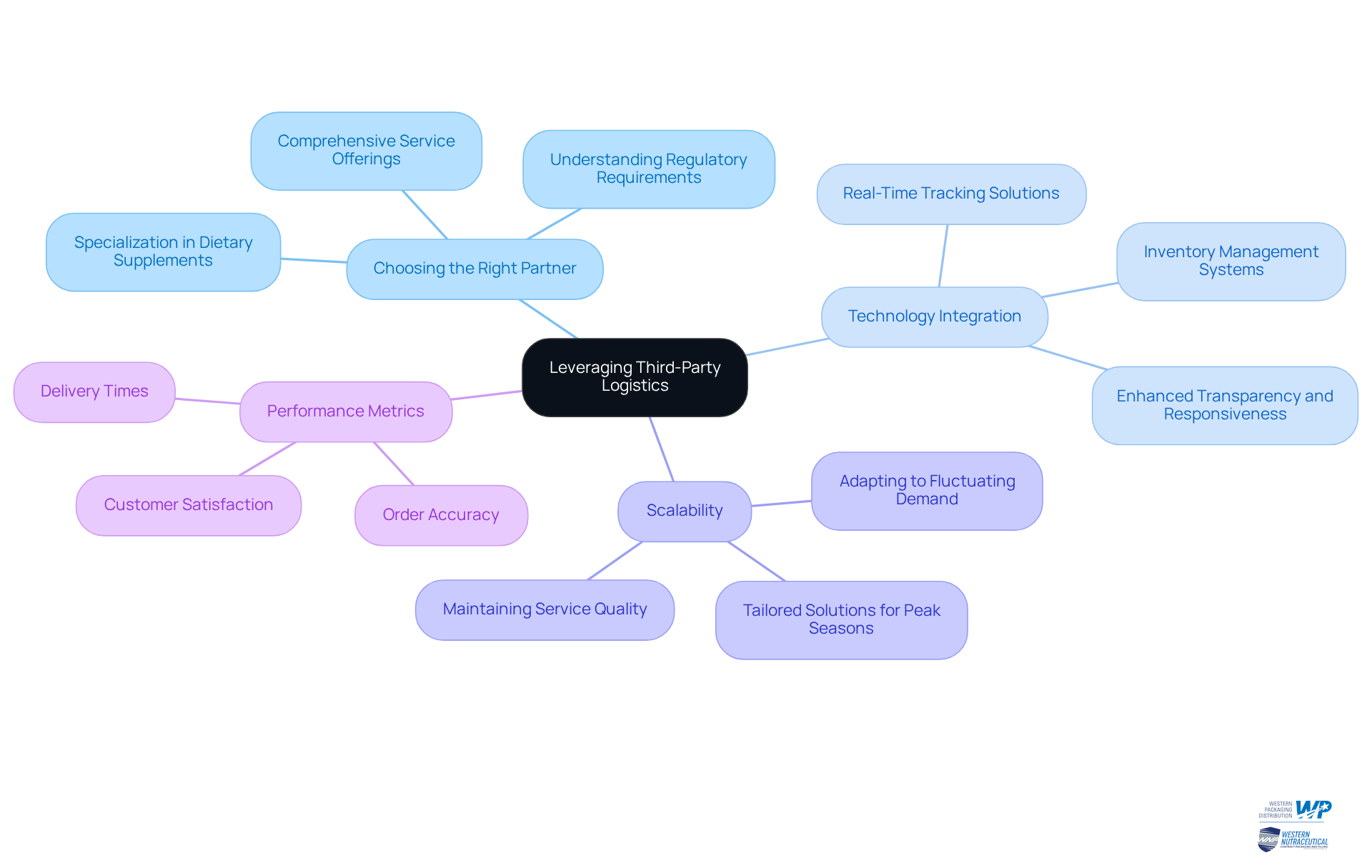
Choosing the Right Partner: Opt for a 3PL provider like Western Packaging, which specializes in dietary supplement packaging. Their comprehensive services ensure they understand regulatory requirements and can manage specialized products effectively.
-
Technology Integration: Implement advanced technology solutions from Western Packaging that facilitate real-time tracking and inventory management. This integration enhances transparency and boosts responsiveness, allowing for swift adjustments to inventory levels and order fulfillment processes.
-
Scalability: Ensure your 3PL partner can scale operations to meet fluctuating demand, especially during peak seasons. Western Packaging offers tailored solutions that adapt to your business needs, ensuring service quality and customer satisfaction.
-
Performance Metrics: Establish clear performance metrics to assess your 3PL provider's effectiveness, focusing on delivery times, order accuracy, and customer satisfaction. With Western Packaging's end-to-end solutions, you can expect high standards in dietary supplement packaging, directly linked to their comprehensive warehousing and logistics services.
By adopting these strategies and partnering with Western Packaging, companies can significantly enhance their distribution capabilities, streamline logistics operations, and improve overall supply chain efficiency.
Conclusion
Mastering dietary supplement packaging transcends mere aesthetics; it embodies a multifaceted approach encompassing regulatory compliance, innovative design, operational efficiency, and strategic logistics. By prioritizing quality standards and adhering to FDA regulations, manufacturers can cultivate trust and confidence among consumers, ensuring their products distinguish themselves in a competitive marketplace.
Key insights from the article underscore the significance of effective packaging design strategies that prioritize clarity, branding consistency, sustainability, and user experience. These elements not only enhance consumer appeal but also resonate with the increasing demand for transparency and eco-friendliness in product offerings. Furthermore, integrating automation and quality control in the filling process can markedly enhance operational efficiency, while leveraging third-party logistics empowers companies to streamline distribution and swiftly adapt to market demands.
In conclusion, the dietary supplement industry is primed for substantial growth, necessitating that companies embrace these best practices to thrive. By concentrating on compliance, innovative packaging, and efficient logistics, businesses can not only meet consumer expectations but also position themselves as leaders in a rapidly evolving market. The future of dietary supplement packaging hinges on a commitment to quality, sustainability, and operational excellence—an endeavor that will ultimately yield lasting success and foster consumer loyalty.
Frequently Asked Questions
What are the key regulations manufacturers must follow for dietary supplements?
Manufacturers must familiarize themselves with the FDA's guidelines, particularly the Current Good Manufacturing Practices (cGMPs) outlined in 21 CFR Part 111.
What are Current Good Manufacturing Practices (cGMPs)?
cGMPs are regulations that ensure dietary supplement packaging materials are safe, suitable, and compliant with labeling requirements.
What steps can manufacturers take to ensure compliance with FDA regulations?
Key steps include conducting regular audits, training staff, maintaining thorough documentation, and consulting experts.
Why is it important to conduct regular audits?
Regular audits help manufacturers review container processes and materials to ensure compliance with FDA regulations.
How can training staff contribute to regulatory compliance?
Training ensures that all employees involved in handling products are knowledgeable about regulatory requirements and quality standards.
What role does documentation play in regulatory compliance?
Maintaining thorough records of all materials and processes demonstrates compliance during inspections.
Why should manufacturers consult experts regarding regulations?
Engaging with regulatory consultants or legal advisors helps companies stay updated on changes in regulations and best practices.
What are the benefits of adhering to quality standards and regulatory compliance?
Adhering to these practices mitigates risks associated with non-compliance and enhances consumer confidence in the products offered.




