Overview
The article delineates best practices for ensuring compliance in OTC packaging for nutraceuticals, underscoring the critical importance of regulatory adherence, effective design strategies, and operational efficiency. By following FDA guidelines, employing consumer-friendly design elements, and integrating filling services with logistics, businesses can significantly enhance product safety, market appeal, and operational performance. This comprehensive approach ultimately fosters consumer trust and satisfaction, establishing a foundation for lasting success in the nutraceutical market.
Introduction
Navigating the complexities of over-the-counter (OTC) packaging compliance is increasingly crucial for nutraceutical companies seeking to excel in a competitive market. Stringent regulations set by the FDA, coupled with a heightened focus on consumer safety, make it essential to comprehend the nuances of packaging requirements. Such understanding can significantly bolster a brand's credibility and market success. As regulations evolve and consumer preferences shift, companies must consider:
- How can they ensure compliance while also captivating their audience?
This article explores best practices for OTC packaging, examining:
- Effective design strategies
- Operational efficiencies
- The pivotal role of compliance in building consumer trust and fostering brand loyalty.
Understand Regulatory Compliance in OTC Packaging
Regulatory compliance in OTC packaging containers is critical for ensuring product safety and market viability. Adhering to guidelines set by the FDA and other regulatory authorities encompasses several key elements:
- Containers must be tamper-evident.
- Labels must be clear and informative.
- All ingredients must be accurately disclosed.
The FDA mandates the use of tamper-proof seals on liquid OTC medications to protect consumers from potential harm. Staying updated on evolving regulations is essential, particularly regarding the FDA's recent guidance on minor alterations to OTC packaging items, which can significantly impact requirements.
Regular training for staff on compliance issues, coupled with systematic audits, can greatly enhance adherence to these regulations. For instance, a nutraceutical company that implemented a comprehensive compliance training program reported a 30% reduction in labeling errors, thereby boosting its market credibility. As noted by an FDA official, "Adherence to Good Manufacturing Practices (GMP) is essential for quality of the item."
Moreover, companies should recognize state-specific regulations, as numerous states are enacting their own labeling laws. Utilizing resources such as the FDA's website and industry associations can provide valuable insights into compliance requirements. Consulting with legal professionals regarding regulations on containers is also advisable to ensure that products meet all essential standards prior to entering the market. This proactive strategy not only enhances compliance but also fosters public trust and safety.
Implement Effective Design Strategies for Consumer Appeal
To develop wrapping that connects with buyers, businesses must emphasize several crucial design components. Color psychology plays a pivotal role in shaping purchasing choices; for instance, green packaging is frequently associated with health and sustainability, attracting environmentally conscious consumers. Research indicates that up to 85% of buyers consider color the primary factor in their selection, underscoring the necessity for strategic color choices.
In addition to color, clear and concise labeling is vital for assisting buyers in swiftly understanding the advantages and applications of the item. Innovative packaging formats, such as resealable pouches or single-serving packets, enhance user experience and cater to the growing trend of on-the-go lifestyles. A notable case study revealed that a leading nutraceutical brand experienced a 25% sales increase within six months after transitioning to resealable pouches, demonstrating the significant impact of functional design.
Moreover, incorporating interactive elements such as QR codes can enhance customer engagement by offering additional product information and nurturing a connection with the brand. By concentrating on design approaches that improve both functionality and aesthetics, companies can considerably boost their market presence and attract contemporary buyers. Furthermore, with an increasing focus on sustainability, it is crucial to consider consumer preferences for environmentally friendly containers, which are expected to be a significant influence in buying choices by 2025.
Trust Western Packaging to deliver innovative packaging solutions that not only comply with industry standards but also enhance your brand recognition and shelf appeal, ensuring a cohesive brand identity across all graphic assets.
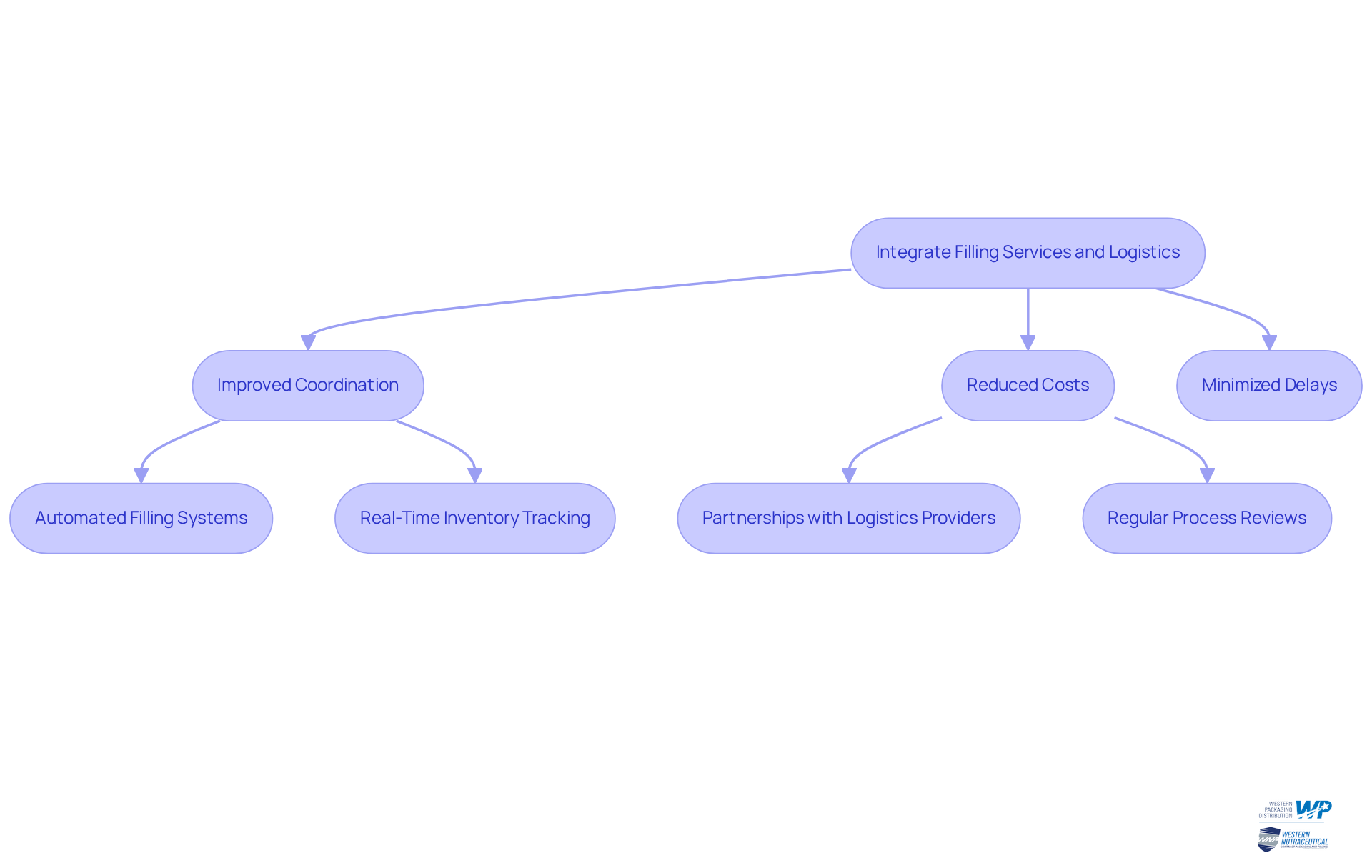
Integrate Filling Services and Logistics for Operational Efficiency
To achieve operational efficiency, businesses should embrace a holistic approach that integrates filling services with logistics, particularly through the comprehensive 3PL services provided by Western Packaging. This strategic integration fosters improved coordination between production and distribution, effectively minimizing delays and reducing costs. For instance, a nutraceutical firm that adopted a unified model reported a remarkable 20% reduction in lead times, enabling them to respond more swiftly to consumer demands. Notably, supply chain integration can cut lead times by as much as 40%, underscoring the substantial advantages of this methodology.
Leveraging advanced technology, such as automated filling systems and real-time inventory tracking, can further amplify efficiency. Companies that have implemented machine learning algorithms have witnessed a 40% enhancement in supply chain management efficiency, illustrating the transformative impact of technology on operational processes. Establishing robust partnerships with logistics providers, including warehousing and inventory management services through Western Packaging's tailored 3PL offerings, is essential to ensure timely delivery of products in optimal condition. As Andrew-vans Bray aptly states, "A partnership relationship between the buyer and the supplier results in higher percentage reduction in lead times compared with adversarial relationships."
Regularly reviewing and refining supply chain processes is vital for identifying bottlenecks and areas ripe for improvement. Common pitfalls include the failure to adapt to evolving market conditions or ineffective communication with partners. By prioritizing integration and remaining cognizant of these challenges, companies can bolster their operational capabilities, ultimately enhancing customer satisfaction and loyalty. Operational efficiency transcends mere speed; it’s about cultivating a seamless flow that aligns with customer expectations and propels business growth.
Conclusion
Navigating the intricate world of OTC packaging compliance is essential for nutraceutical companies aiming to thrive in today's competitive landscape. Understanding and implementing best practices not only ensures adherence to stringent regulations but also enhances brand credibility and fosters consumer trust.
This article highlights several critical components for successful OTC packaging, including:
- The necessity of regulatory compliance
- Effective design strategies that resonate with consumers
- The integration of filling services and logistics for operational efficiency
Key insights demonstrate the importance of:
- Tamper-evident containers
- Clear labeling
- Innovative packaging formats that cater to modern consumer preferences
Furthermore, leveraging technology and maintaining robust partnerships can significantly improve supply chain management and reduce lead times.
Ultimately, the significance of mastering OTC packaging extends beyond compliance—it is about creating a trustworthy brand that resonates with consumers and meets their evolving demands. Companies are encouraged to:
- Stay informed about current regulations and trends
- Invest in efficient operational practices
- Prioritize design strategies that enhance consumer appeal
By doing so, businesses can not only meet regulatory requirements but also position themselves for sustained success in the nutraceutical market.
Frequently Asked Questions
Why is regulatory compliance important in OTC packaging?
Regulatory compliance in OTC packaging is crucial for ensuring product safety and market viability, as it adheres to guidelines set by the FDA and other regulatory authorities.
What are the key elements of regulatory compliance in OTC packaging?
Key elements include the use of tamper-evident containers, clear and informative labels, and accurate disclosure of all ingredients.
What specific requirement does the FDA mandate for liquid OTC medications?
The FDA mandates the use of tamper-proof seals on liquid OTC medications to protect consumers from potential harm.
How can companies stay updated on regulatory changes?
Companies should stay updated on evolving regulations, particularly regarding the FDA's guidance on minor alterations to OTC packaging, which can significantly impact compliance requirements.
What strategies can enhance adherence to regulatory compliance?
Regular training for staff on compliance issues and conducting systematic audits can greatly enhance adherence to regulations.
Can you provide an example of how compliance training has benefited a company?
A nutraceutical company that implemented a comprehensive compliance training program reported a 30% reduction in labeling errors, which boosted its market credibility.
What role do Good Manufacturing Practices (GMP) play in regulatory compliance?
Adherence to Good Manufacturing Practices (GMP) is essential for ensuring the quality of OTC products.
Are there additional regulations that companies should consider?
Yes, companies should recognize state-specific regulations, as many states are enacting their own labeling laws.
Where can companies find resources regarding compliance requirements?
Companies can utilize resources such as the FDA's website and industry associations for valuable insights into compliance requirements.
Is it advisable to consult legal professionals regarding packaging regulations?
Yes, consulting with legal professionals about regulations on containers is advisable to ensure that products meet all essential standards before entering the market.




