Overview
This article delves into best practices for package manufacturing within the nutraceuticals industry, underscoring the critical role of quality management systems, regulatory compliance, and supply chain optimization. By implementing rigorous quality standards, navigating complex regulatory requirements, and adopting advanced inventory management systems, companies can ensure product safety, compliance, and operational efficiency in a rapidly expanding market. These strategies are essential for positioning businesses as reliable leaders in the industry, ultimately driving success and customer trust.
Introduction
In a rapidly evolving market, the nutraceutical packaging industry confronts the dual challenge of ensuring product integrity while navigating complex regulatory landscapes. Companies are compelled to meet consumer demands for quality and safety, making the understanding and implementation of best practices in package manufacturing essential.
What strategies can nutraceutical manufacturers adopt to not only comply with regulations but also enhance operational efficiency and product safety? This article explores key practices that can elevate packaging standards and drive success in this competitive sector.
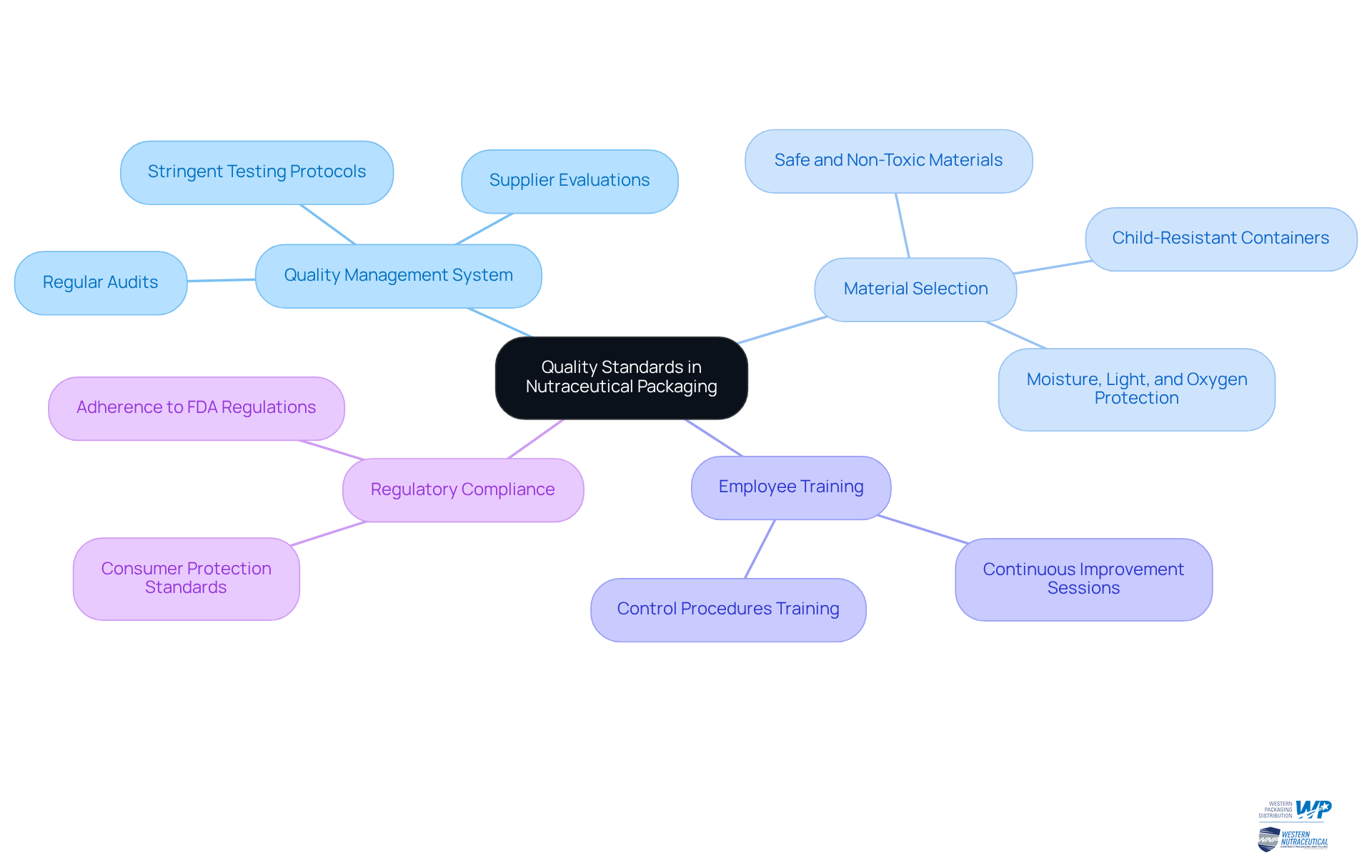
Establish Quality Standards in Nutraceutical Packaging
To establish robust quality standards in nutraceutical containers, companies must implement a comprehensive quality management system (QMS) that encompasses:
- Regular audits
- Supplier evaluations
- Stringent testing protocols
This system should prioritize the selection of materials that are safe, non-toxic, and tailored to the specific product type. For instance, using child-resistant containers for supplements intended for children significantly enhances safety and compliance with regulatory standards.
Moreover, it is essential to consider the shelf life of items by selecting materials that effectively protect against moisture, light, and oxygen—elements known to compromise product integrity. Consistent training sessions for employees on control procedures are vital, ensuring that all team members involved in packaging understand their responsibilities in maintaining these standards.
The package manufacturing industry for dietary supplements is projected to grow at a CAGR of 5.6% between 2025 and 2030, underscoring the importance of management standards in a burgeoning market. Successful examples include companies that have adopted automated inspection technologies, resulting in notable improvements in compliance with standards. By fostering a culture of excellence and continuous improvement, nutraceutical companies can enhance product safety and consumer trust, ultimately driving market success. Additionally, adherence to regulatory standards is crucial for maintaining quality and ensuring consumer protection.
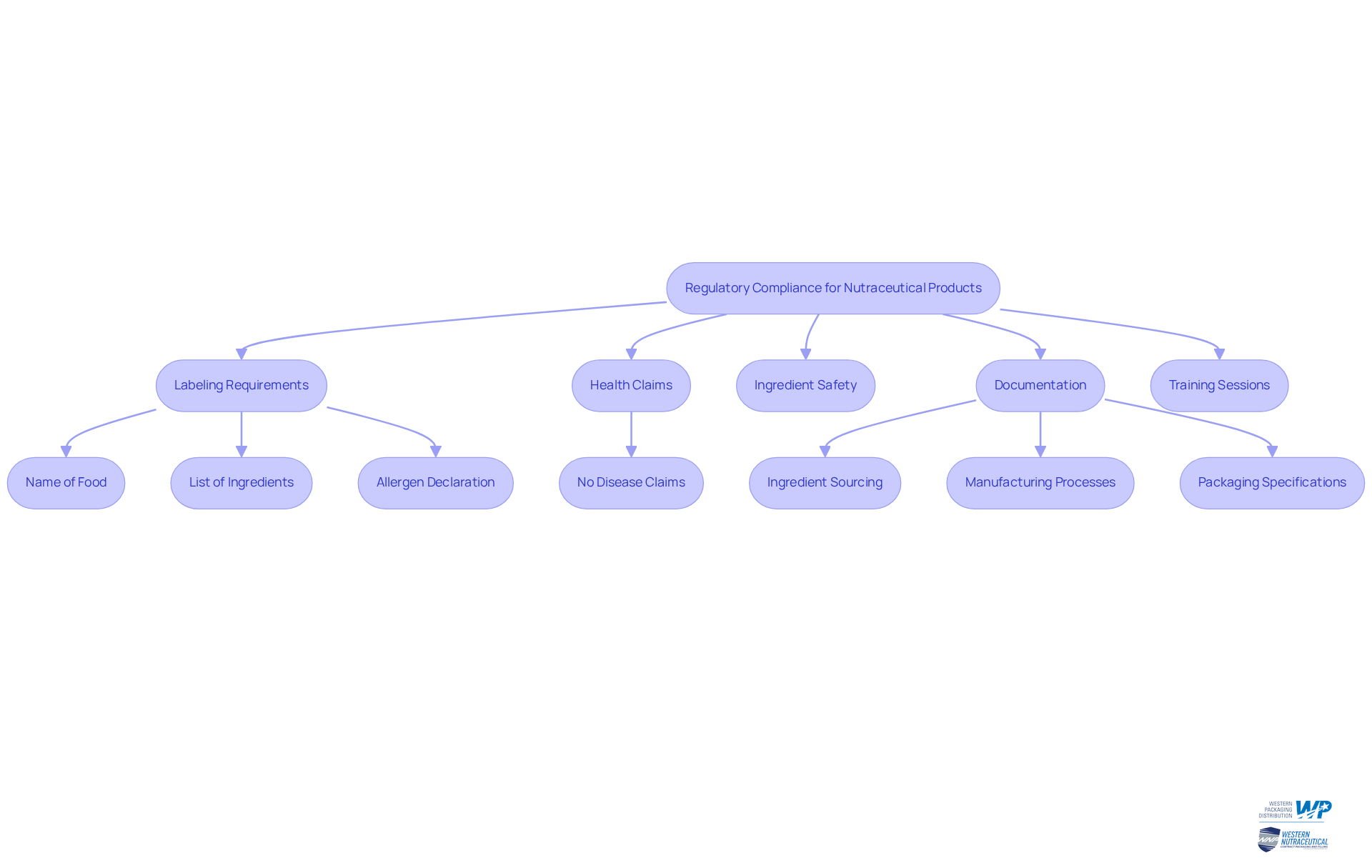
Navigate Regulatory Compliance for Nutraceutical Products
To navigate regulatory adherence effectively, companies must remain vigilant about the latest regulations from authoritative bodies such as the FDA and FTC. This vigilance encompasses a thorough understanding of:
- Labeling requirements
- Health claims
- Ingredient safety
Crucially, health supplements must not assert that they prevent, treat, or cure diseases, as this is a fundamental regulatory requirement. Collaborating with regulatory advisors can provide invaluable insights and assist in optimizing the adherence process. Furthermore, brands should create a claim substantiation document to validate the efficacy and safety of their products, ensuring that all health claims are substantiated by scientific literature.
Establishing a robust documentation system is essential for monitoring adherence efforts, which should include:
- Ingredient sourcing
- Package manufacturing processes
- Packaging specifications
Regular training sessions for employees on adherence matters are vital in guaranteeing that everyone comprehends their responsibilities and the importance of compliance with regulations. For instance, the Food Safety and Standards (Labelling and Display) Regulations, 2020, mandate that labels include crucial information such as the name of the food, a list of ingredients, and a declaration of allergens.
Consider the example of a nutraceutical company that adeptly navigated a complex labeling requirement, enabling it to launch its product on schedule while avoiding delays and potential fines. This case underscores the necessity of proactive regulatory strategies in maintaining operational efficiency and market readiness. As the industry continues to evolve, remaining informed and prepared will be essential to overcoming compliance challenges and achieving long-term success.
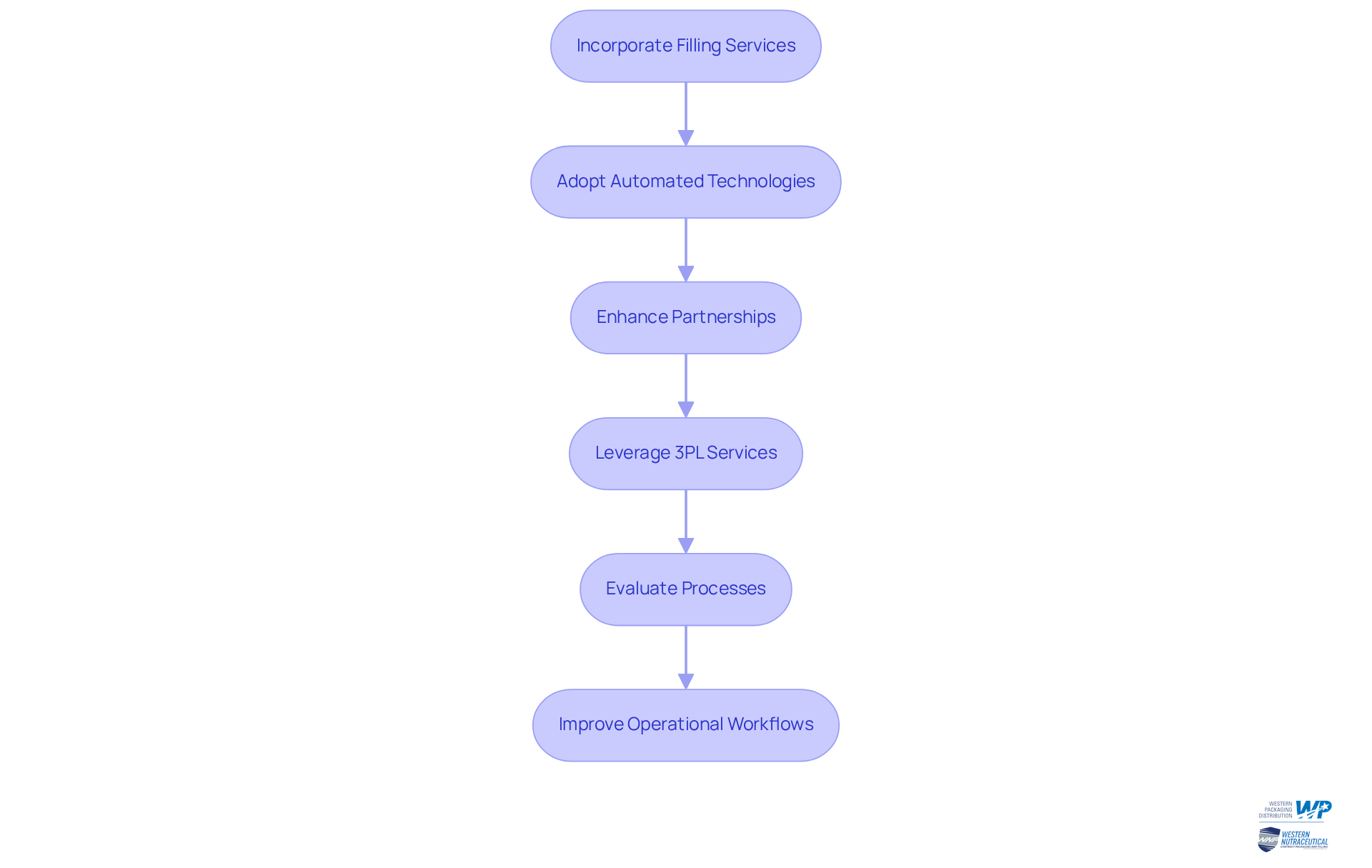
Integrate Filling Services for Streamlined Operations
Incorporating filling services into package manufacturing processes is essential for enhancing production efficiency in the dietary supplement industry. Companies must prioritize adopting automated filling technologies in package manufacturing that are capable of handling diverse product types, including powders, gummies, and soft gels. This approach not only accelerates the filling process in package manufacturing but also minimizes the risk of human error, which is vital for maintaining product integrity.
Establishing strong partnerships with filling service providers enhances communication and coordination, ensuring that production schedules align seamlessly with package manufacturing needs. Furthermore, leveraging comprehensive 3PL services from Western Packaging can optimize package manufacturing and enhance supply chain management.
For instance, a health product manufacturer that adopted an automated filling line experienced an impressive 30% decrease in production time, enabling them to react swiftly to market needs. Regular evaluations of the filling process in package manufacturing are crucial for identifying opportunities for improvement, ensuring that operations remain efficient and cost-effective. By embracing these strategies, companies can significantly enhance their operational workflows in package manufacturing and better meet the evolving needs of consumers.
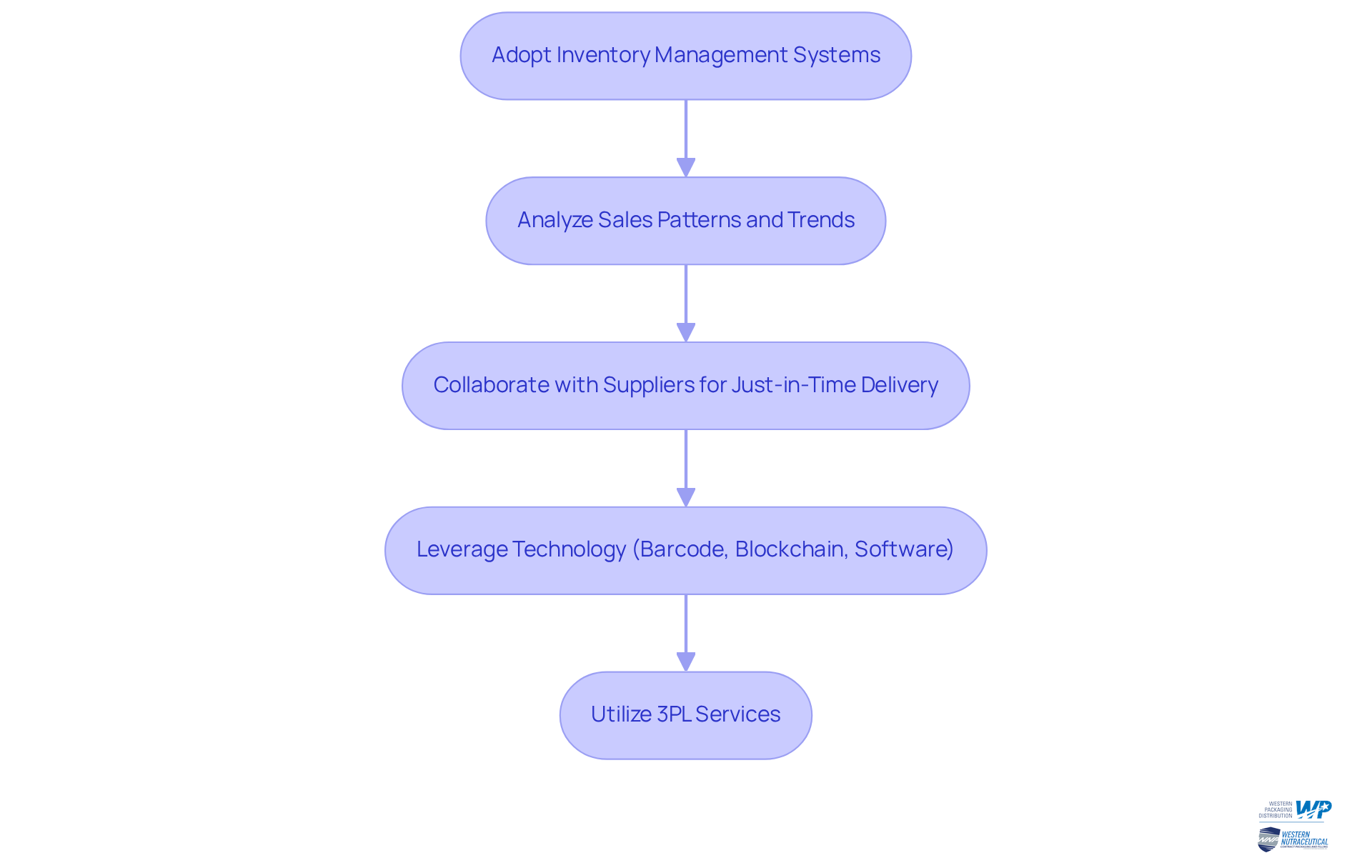
Optimize Supply Chain Management for Packaging Efficiency
To enhance supply chain management, businesses in the health supplement sector must adopt inventory management systems that provide real-time information on stock levels and demand predictions. This proactive approach facilitates better planning, significantly reducing the risk of overstocking or stockouts.
Analyzing sales patterns and seasonal trends is crucial for achieving the right balance of inventory, ensuring businesses can effectively meet customer demand. Collaborating with suppliers to establish just-in-time delivery systems can further enhance efficiency, guaranteeing that materials arrive precisely when needed without excess inventory.
Moreover, leveraging technology such as barcode systems, blockchain, and inventory management software can improve organization and tracking within the supply chain, which is increasingly vital in the dietary supplement sector.
Western Packaging offers comprehensive 3PL services, including tailored warehousing solutions that optimize storage space and logistics services to ensure timely distribution for package manufacturing. By utilizing these services, nutraceutical manufacturers can streamline their operations and concentrate on core competencies while reducing holding costs and enhancing their ability to meet customer demand promptly.
For instance, a company that implemented a just-in-time inventory system reduced its holding costs by 25% while improving its responsiveness to customer demand. As Peter Drucker famously stated, 'What Gets Measured, Gets Managed,' underscoring the importance of tracking inventory for effective management.
Conclusion
Implementing best practices in nutraceutical packaging is essential for ensuring product quality, regulatory compliance, and operational efficiency. By focusing on establishing a comprehensive quality management system, companies can enhance consumer trust and product safety. This foundational approach not only addresses the immediate needs of packaging but also positions businesses for long-term success in a rapidly growing market.
Key strategies include:
- The importance of stringent quality standards
- Navigating complex regulatory requirements
- Integrating automated filling services
- Optimizing supply chain management
Each component plays a critical role in maintaining the integrity of nutraceutical products while meeting consumer demands. For instance, adopting automated technologies can significantly reduce production time and errors, while effective inventory management can streamline operations and minimize costs.
As the nutraceutical industry continues to evolve, embracing these best practices will be vital for companies aiming to stay competitive. By prioritizing quality, compliance, and efficiency, businesses can not only meet current market demands but also anticipate future trends, ultimately driving growth and innovation in the nutraceutical packaging sector. Taking proactive steps today will ensure that companies are well-equipped to thrive in the dynamic landscape of health supplements.
Frequently Asked Questions
What is essential for establishing quality standards in nutraceutical packaging?
Establishing quality standards requires implementing a comprehensive quality management system (QMS) that includes regular audits, supplier evaluations, and stringent testing protocols.
Why is the selection of materials important in nutraceutical packaging?
The selection of materials is crucial because they must be safe, non-toxic, and suitable for the specific product type, such as using child-resistant containers for supplements intended for children to enhance safety and compliance with regulatory standards.
How does shelf life impact the choice of packaging materials?
Packaging materials must effectively protect against moisture, light, and oxygen to maintain the integrity of the product and ensure an adequate shelf life.
What role does employee training play in maintaining quality standards?
Consistent training sessions for employees on control procedures are vital to ensure that all team members involved in packaging understand their responsibilities in maintaining quality standards.
What is the projected growth rate for the package manufacturing industry for dietary supplements?
The package manufacturing industry for dietary supplements is projected to grow at a CAGR of 5.6% between 2025 and 2030.
How can companies improve compliance with quality standards?
Companies can improve compliance by adopting automated inspection technologies, which have shown notable improvements in adhering to standards.
Why is adherence to regulatory standards important in nutraceutical packaging?
Adherence to regulatory standards is crucial for maintaining quality and ensuring consumer protection, which ultimately enhances product safety and consumer trust.




