Overview
This article delves into the mastery of primary and secondary packaging within the nutraceuticals sector, highlighting their crucial roles in product protection and regulatory compliance. Effective packaging choices—such as barrier properties, sustainability, and safety features—are essential for maintaining product integrity and fulfilling consumer expectations. These factors not only enhance brand trust but also bolster market competitiveness. By understanding and implementing these strategies, businesses can ensure that their packaging meets the highest standards, fostering reliability and confidence among consumers.
Introduction
In the rapidly evolving nutraceutical industry, the significance of effective packaging cannot be overstated. Primary and secondary packaging not only protects products but also plays a crucial role in shaping consumer perception and ensuring regulatory compliance. As businesses navigate the complexities of material selection, sustainability, and adherence to stringent regulations, they face the challenge of balancing quality with cost-effectiveness.
What strategies can companies employ to master the art of packaging while ensuring safety, compliance, and market appeal?
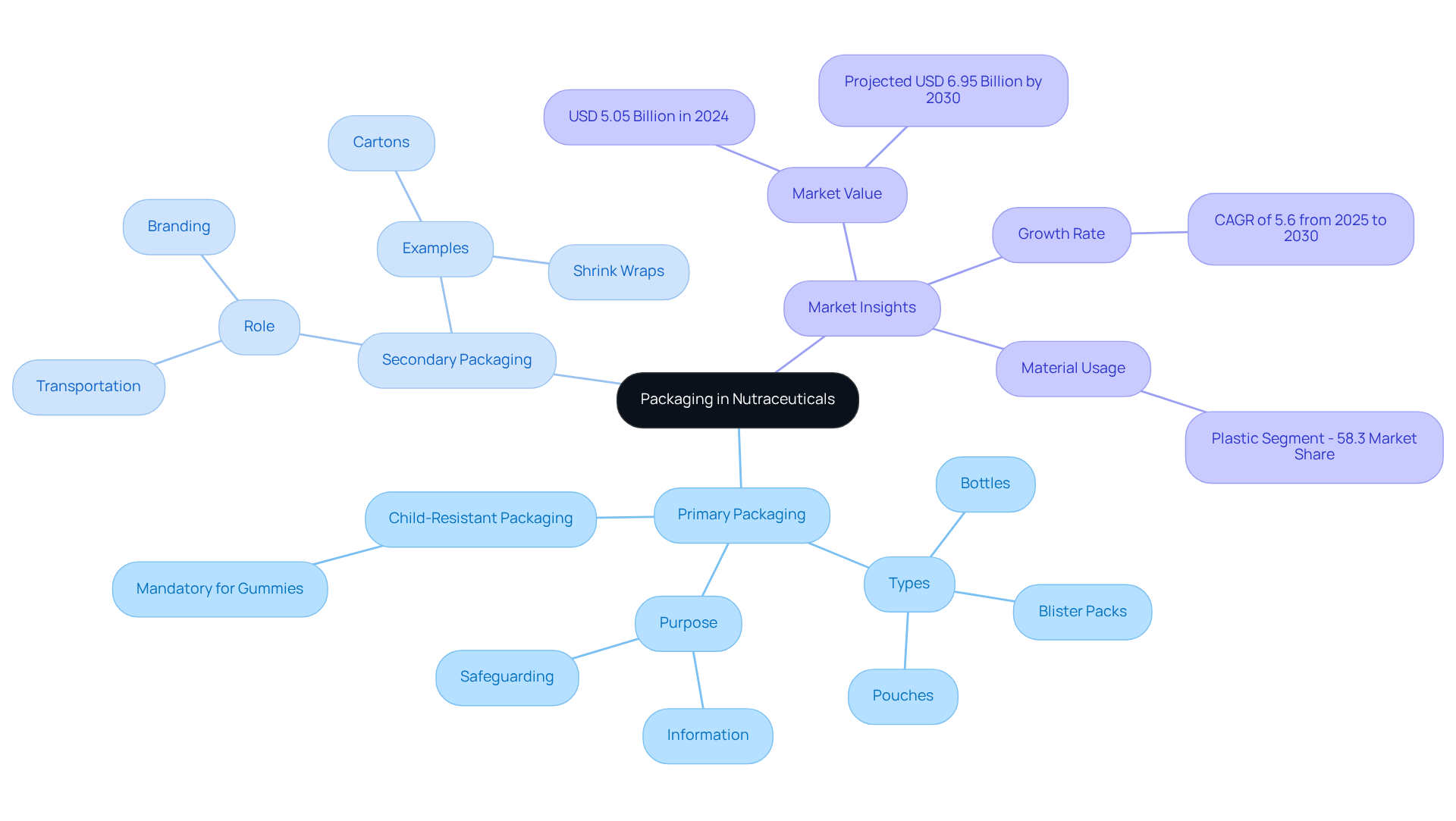
Define Primary and Secondary Packaging in Nutraceuticals
In the nutraceutical sector, primary and secondary packaging refers to the immediate vessel that holds the item, which can include bottles, blister packs, or pouches. Its primary purpose is to safeguard the item from contamination and deterioration while providing essential information to consumers.
For instance, child-resistant containers (CRP) are a necessary condition for items like gummies, ensuring safety for children. Conversely, secondary wrapping is part of the primary and secondary packaging process, as it encompasses the outer layer that groups multiple primary packages together, such as cartons or shrink wraps. This layer not only offers additional protection during transportation but also plays a vital role in branding and compliance with regulatory standards.
According to industry specialists, effective primary containment is essential for preserving the quality and efficacy of nutraceuticals, as it directly impacts consumer trust and product appeal. Understanding these definitions is crucial for making informed choices throughout the wrapping process, particularly as the global nutraceutical wrapping market was valued at approximately USD 5.05 billion in 2024 and is projected to expand at a compound annual growth rate of 5.6% from 2025 to 2030, achieving around USD 6.95 billion by 2030.
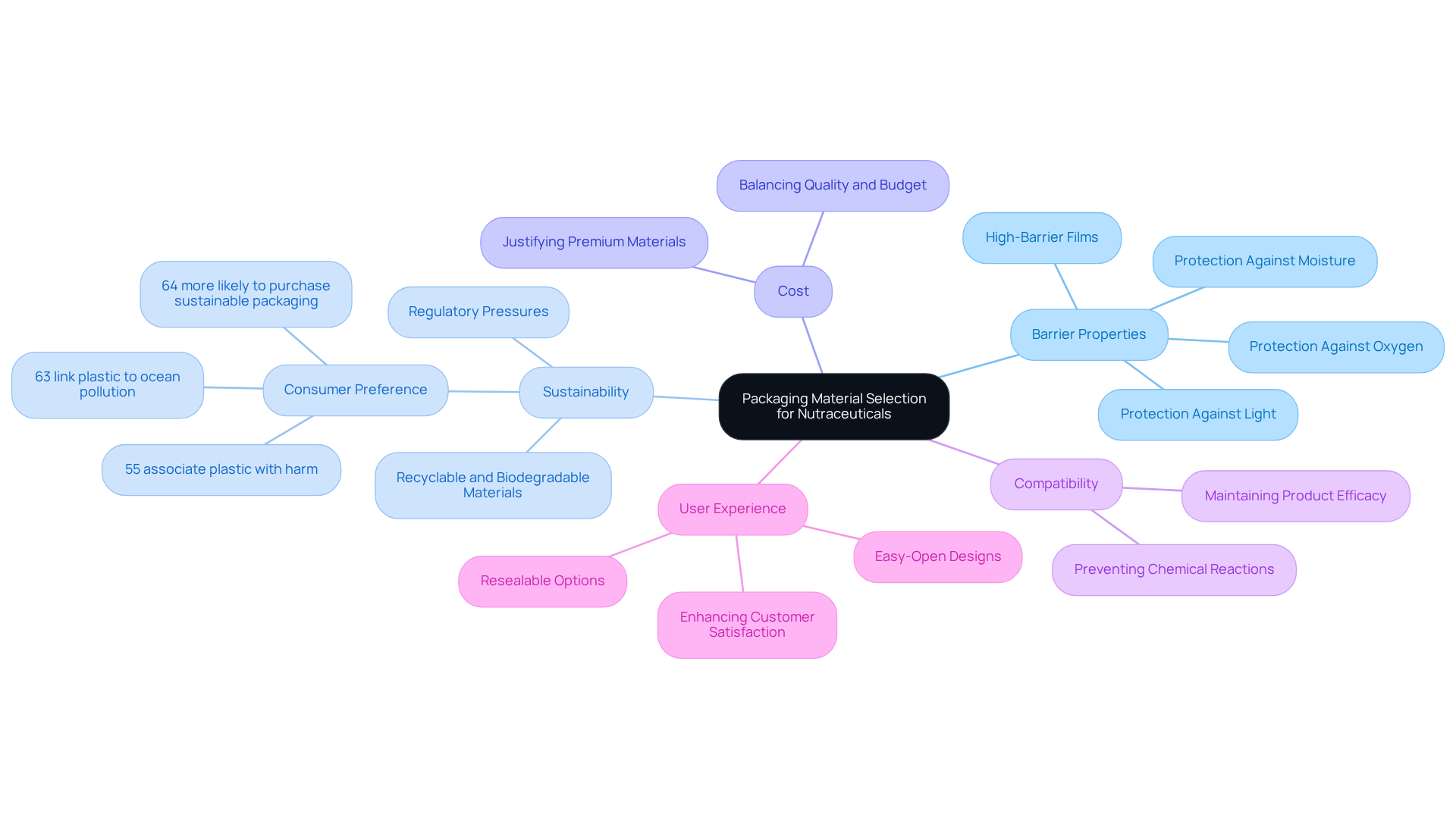
Evaluate Key Factors for Packaging Material Selection
When selecting packaging materials for nutraceuticals, several key factors must be considered:
-
Barrier Properties: Choosing materials that effectively protect against moisture, light, and oxygen is essential, as these elements can significantly diminish quality. High-barrier films, for instance, are particularly suitable for sensitive ingredients, ensuring their integrity throughout the product's shelf life.
-
Sustainability: With growing public interest in environmentally friendly choices, integrating recyclable or biodegradable materials into containers is crucial. This approach not only meets customer expectations but also aligns with increasing regulatory pressures for sustainable practices. In fact, 64% of buyers are more likely to purchase from brands that utilize sustainable packaging, reflecting a significant change in purchasing behavior. Furthermore, 55% of consumers associate plastic with being harmful, and 63% link it to ocean pollution, underscoring the urgency for sustainable alternatives.
-
Cost: Striking a balance between quality and budget constraints is vital. While premium materials may offer enhanced protection, their application should be justified by the item's market positioning and potential return on investment.
-
Compatibility: The selected packaging material must be compatible with the formulation to prevent any chemical reactions that could undermine efficacy. This compatibility is essential for maintaining the product's intended benefits.
-
User Experience: Enhancing customer satisfaction through user-friendly features, such as resealable options or easy-open designs, fosters brand loyalty. As consumer preferences evolve, containers that emphasize convenience are increasingly appreciated.
By addressing these factors, businesses can enhance their distribution strategies to meet both market demands and sustainability objectives, ultimately improving their competitive advantage in the health supplement sector. Furthermore, the health product rigid containers market is expected to expand at a CAGR of 6.3% from 2025 to 2035, emphasizing the significance of adjusting to these trends.
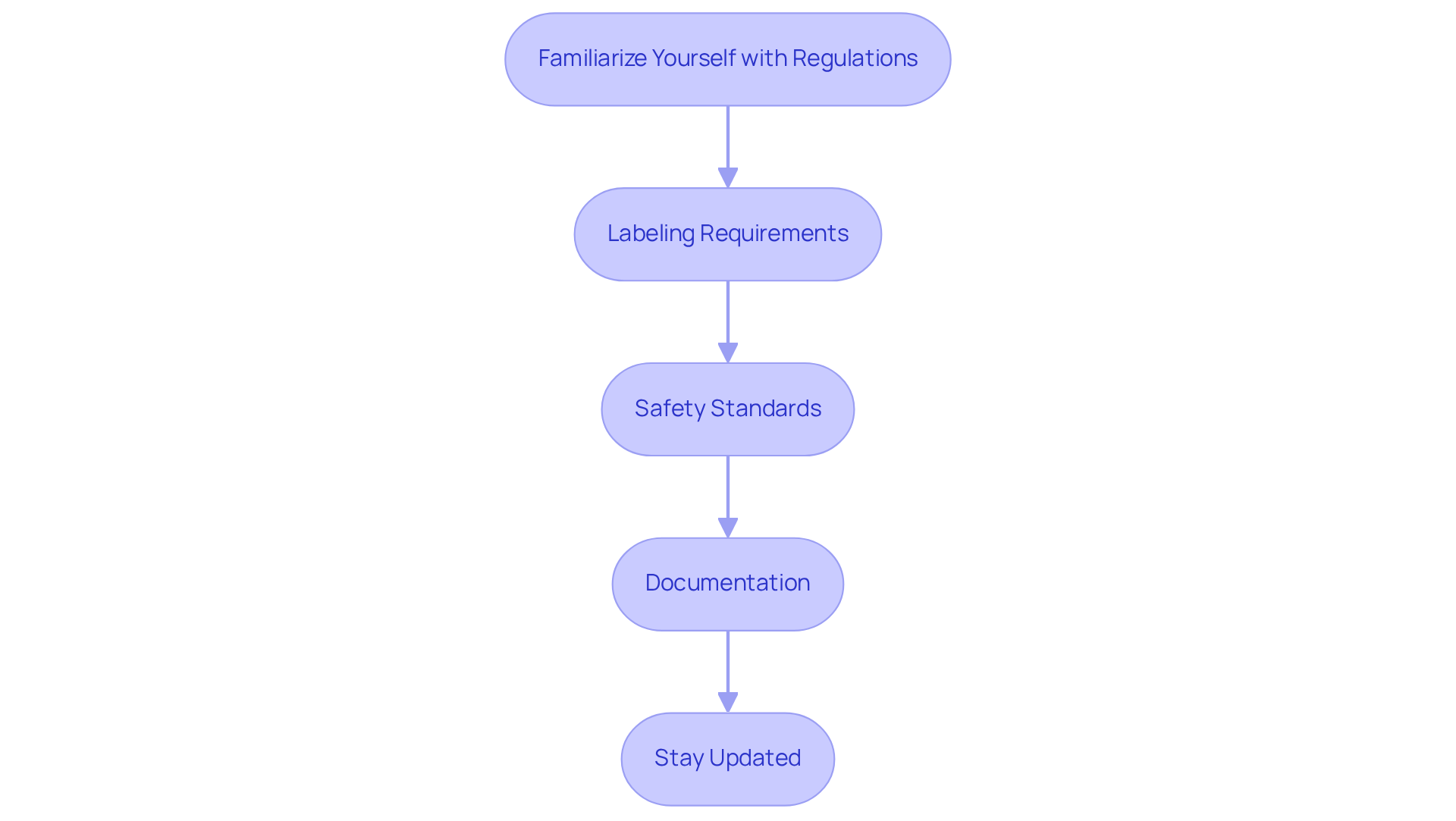
Ensure Compliance with Regulatory Standards
To ensure compliance with regulatory standards in nutraceutical packaging, it is essential to follow these key steps:
-
Familiarize Yourself with Regulations: Understanding the specific regulations that pertain to your category, including FDA guidelines for dietary supplements, is crucial. Since nutraceuticals were officially classified by the FDA in 1994, staying informed about these regulations is paramount for compliance.
-
Labeling Requirements: Ensure that all labels include necessary information such as ingredient lists, nutritional facts, and any health claims. Labels must be clear, accurate, and not misleading. Compliant containers can enhance buyer trust by 28%, making adherence to labeling standards essential. Incorrect labeling remains a frequent error in the nutraceutical industry, leading to severe penalties and product recalls.
-
Safety Standards: Use materials that are approved for food contact and free from harmful substances, such as BPA. Conduct safety assessments to validate compliance. Attributes such as tamper-evident seals and child-resistant closures are crucial for safety and regulatory adherence, as they can raise container costs by 17% but significantly improve consumer trust. Moreover, utilizing tamper-evident pouches for gummies can increase repeat purchases by 15%, demonstrating the commercial advantages of safety features in containers.
-
Documentation: Maintain thorough records of all materials and processes to demonstrate compliance during audits or inspections. Records must be archived for a minimum of six years, ensuring traceability and proof of compliance during inspections.
-
Stay Updated: Regulatory standards can change, so regularly reviewing updates from relevant authorities is essential for ongoing compliance. With 25% of health supplement brands encountering compliance violations in 2024, subscribing to industry newsletters and participating in webinars is vital to remain updated on changing regulations.
By following these steps, supplement producers can effectively manage the intricacies of compliance, ensuring their products meet both safety and regulatory standards.
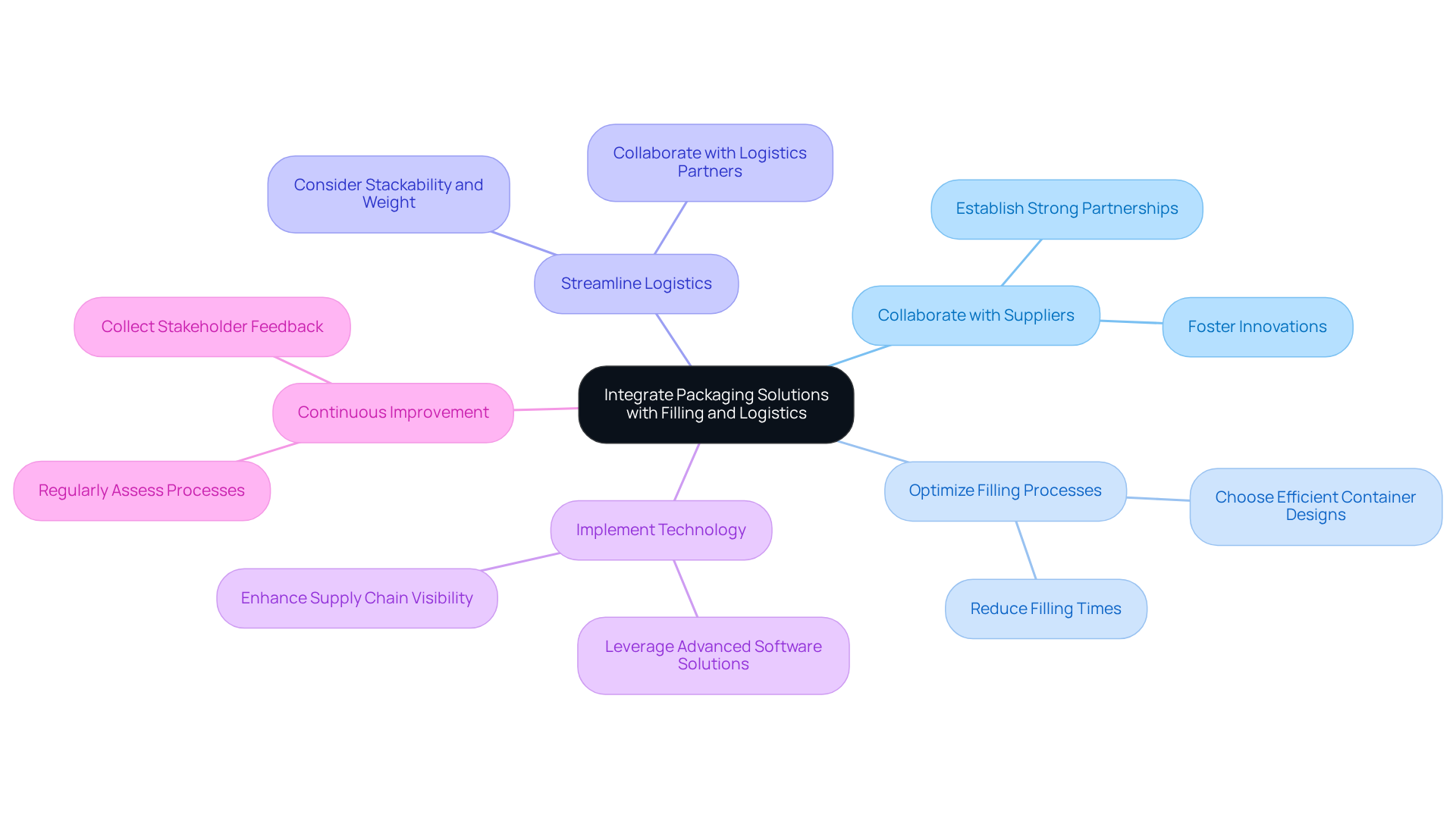
Integrate Packaging Solutions with Filling and Logistics
To effectively integrate packaging solutions with filling and logistics in the nutraceutical sector, consider the following strategies:
-
Collaborate with Suppliers: Establish strong partnerships with material providers to ensure that resources align with your filling processes. This collaboration can foster innovations that significantly enhance operational efficiency. As Aditi Shivarkar observes, collaboration is essential for promoting progress in container solutions that satisfy market needs.
-
Optimize Filling Processes: Choose container designs that encourage quick and accurate filling. For instance, pre-formed pouches can drastically reduce filling times and minimize waste, contributing to a more streamlined operation. The nutraceutical container market is forecasted to expand from USD 3.52 billion in 2024 to USD 4.66 billion by 2030, underscoring the significance of effective processes in a competitive environment.
-
Streamline Logistics: Collaborate closely with logistics partners to create solutions that maximize efficiency in storage and transportation. Key considerations include stackability and weight, which can lead to reduced shipping costs and improved logistics performance. Notably, firms such as Amcor and Gerresheimer AG are at the forefront of creating solutions that enhance logistics efficiency.
-
Implement Technology: Leverage advanced software solutions for inventory management and tracking to enhance supply chain visibility. This approach aids in anticipating demand and effectively managing stock levels, ensuring that operations remain agile and responsive.
-
Continuous Improvement: Regularly assess the integration of wrapping, filling, and logistics processes to identify opportunities for enhancement. Collect feedback from stakeholders to refine operations, ensuring that efficiency and effectiveness are consistently improved. The trend towards sustainable packaging solutions, particularly in primary and secondary packaging, is also gaining traction, reflecting consumer preferences and regulatory pressures, which should be considered in ongoing assessments.
Conclusion
Mastering the intricacies of primary and secondary packaging in nutraceuticals is essential for ensuring product safety, compliance, and consumer satisfaction. Understanding the distinctions between these packaging types not only helps safeguard the product from contamination but also plays a pivotal role in enhancing brand trust and market appeal. As the nutraceutical industry continues to grow, the importance of effective packaging strategies that align with consumer preferences and regulatory demands becomes increasingly significant.
Key factors such as:
- Barrier properties
- Sustainability
- Cost
- Compatibility
- User experience
must be carefully evaluated when selecting packaging materials. By prioritizing these elements, businesses can enhance their distribution strategies, meet market demands, and improve their competitive edge in a rapidly evolving sector. Additionally, adherence to regulatory standards and a commitment to continuous improvement in logistics and filling processes are crucial for maintaining compliance and operational efficiency.
Ultimately, the significance of well-designed packaging solutions extends beyond mere functionality; it reflects a broader commitment to consumer safety, environmental stewardship, and quality assurance. As the nutraceutical market continues to expand, embracing innovative packaging practices will not only meet current consumer expectations but also pave the way for future growth and sustainability in the industry. The time to act is now—invest in packaging solutions that not only protect your product but also enhance your brand's reputation and consumer trust.
Frequently Asked Questions
What is primary packaging in nutraceuticals?
Primary packaging refers to the immediate vessel that holds the nutraceutical item, such as bottles, blister packs, or pouches. Its main purpose is to protect the item from contamination and deterioration while providing essential information to consumers.
What is secondary packaging in nutraceuticals?
Secondary packaging is the outer layer that groups multiple primary packages together, such as cartons or shrink wraps. It provides additional protection during transportation and plays a vital role in branding and compliance with regulatory standards.
Why are child-resistant containers important in nutraceutical packaging?
Child-resistant containers (CRP) are important for items like gummies to ensure safety for children and prevent accidental ingestion.
How does primary packaging affect consumer trust in nutraceuticals?
Effective primary containment is essential for preserving the quality and efficacy of nutraceuticals, which directly impacts consumer trust and product appeal.
What was the value of the global nutraceutical wrapping market in 2024, and what is its projected growth?
The global nutraceutical wrapping market was valued at approximately USD 5.05 billion in 2024 and is projected to expand at a compound annual growth rate of 5.6% from 2025 to 2030, reaching around USD 6.95 billion by 2030.




