Overview
The article emphasizes essential practices for tablet packaging within the nutraceutical industry, highlighting the critical aspects of:
- Item protection
- Dosage accuracy
- User convenience
- Regulatory compliance
- Sustainability
These practices are underpinned by comprehensive discussions on:
- Material selection
- Labeling standards
- Quality control measures
Collectively, they enhance product integrity, bolster consumer trust, and drive market success. By adhering to these guidelines, industry professionals can ensure that their packaging solutions not only meet regulatory demands but also resonate with consumers, ultimately leading to greater market competitiveness.
Introduction
In the competitive landscape of nutraceuticals, the significance of effective tablet packaging is paramount. Manufacturers are compelled to meet consumer demands for safety, convenience, and sustainability, making it essential to understand the best practices in tablet packaging.
What challenges do companies encounter when balancing regulatory compliance with innovative design? Moreover, how can they ensure their products not only stand out on the shelf but also maintain integrity and efficacy?
This exploration delves into best practices that enhance product appeal while safeguarding consumer trust in an ever-evolving market.
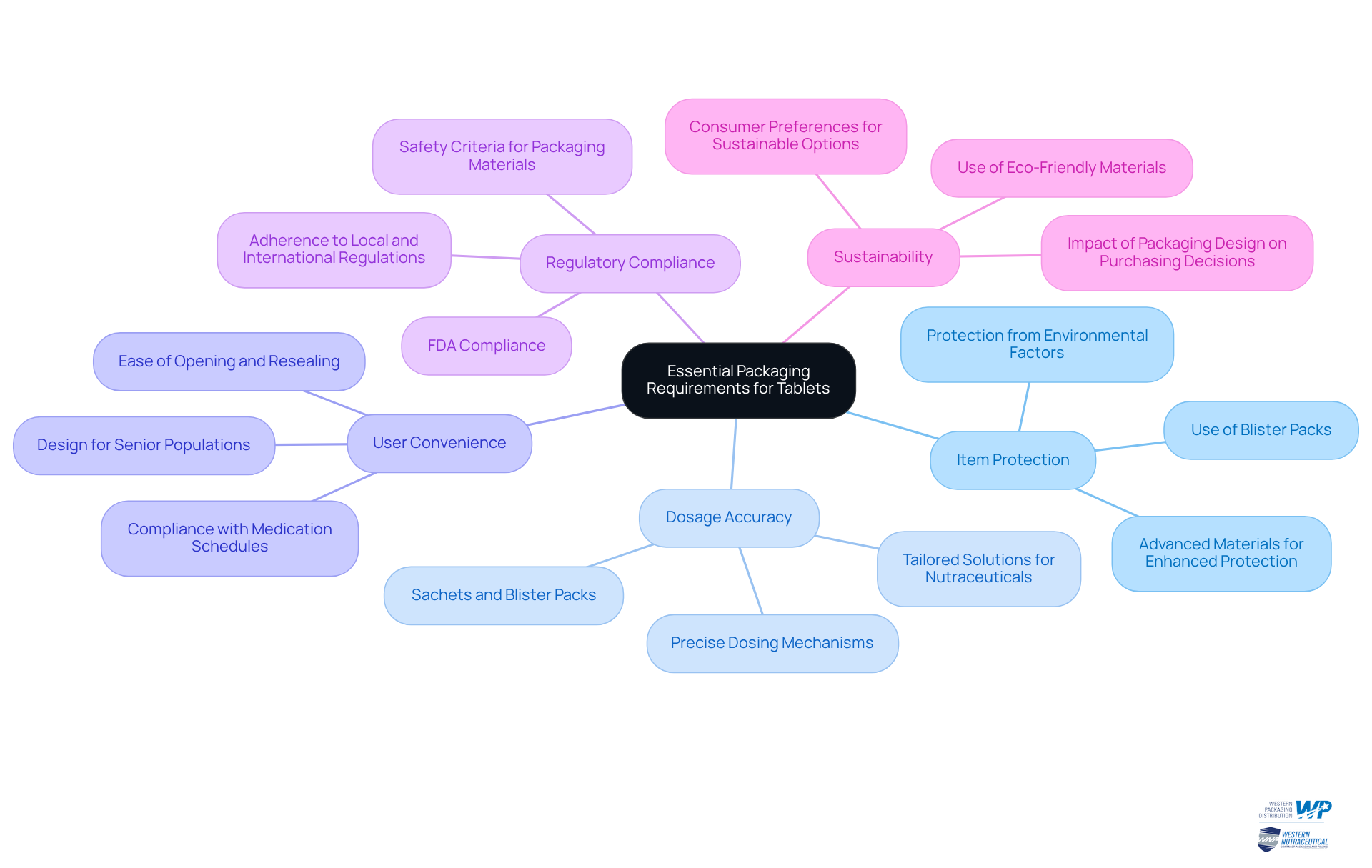
Establish Essential Packaging Requirements for Tablets
To establish essential packaging requirements for tablets, manufacturers must prioritize several critical factors:
-
Item Protection: Packaging must effectively shield tablets from environmental factors such as moisture, light, and oxygen, which can compromise integrity. Tablet packaging, like blister packs, is designed to protect medications from these elements, ensuring their potency and safety. Western Packaging's innovative flexible solutions utilize advanced materials that enhance item protection while elevating brand recognition.
-
Dosage Accuracy: Containers must facilitate precise dosing, particularly for products requiring exact measurements. Sachets and blister packs can enhance dosing accuracy, making it easier for patients to administer the correct amount. Our tailored solutions ensure that each package meets the specific needs of nutraceutical manufacturers, incorporating features that simplify dosage.
-
User Convenience: The end-user experience is paramount; the container should be easy to open and reseal, significantly enhancing customer satisfaction. User-friendly designs in tablet packaging, like those provided by Western Packaging, enhance compliance with medication schedules, particularly for senior populations who may find intricate containers challenging. For instance, our blister containers are designed for easy access, ensuring that patients can manage their medications effectively.
-
Regulatory Compliance: Manufacturers must be well-versed in local and international regulations regarding container materials and labeling. Adherence guarantees that containers meet safety criteria and prevents possible legal complications, protecting both the producer and the buyer. Western Packaging prioritizes compliance with primary container regulations, ensuring that our solutions are designed to meet FDA requirements.
-
Sustainability: With an increasing public preference for eco-friendly options, it is essential to consider sustainable materials that align with market trends. Employing recyclable or biodegradable materials can lessen environmental impact while connecting with eco-aware individuals. Western Packaging is dedicated to offering eco-friendly solutions that improve item attractiveness while fulfilling customer expectations. Indeed, 72% of American buyers indicated that their purchasing choices were affected by an item's design, emphasizing the significance of sustainability in container selections.
By addressing these requirements, manufacturers can create tablet packaging that not only protects the product but also appeals to consumers and meets regulatory standards, ultimately enhancing market success.
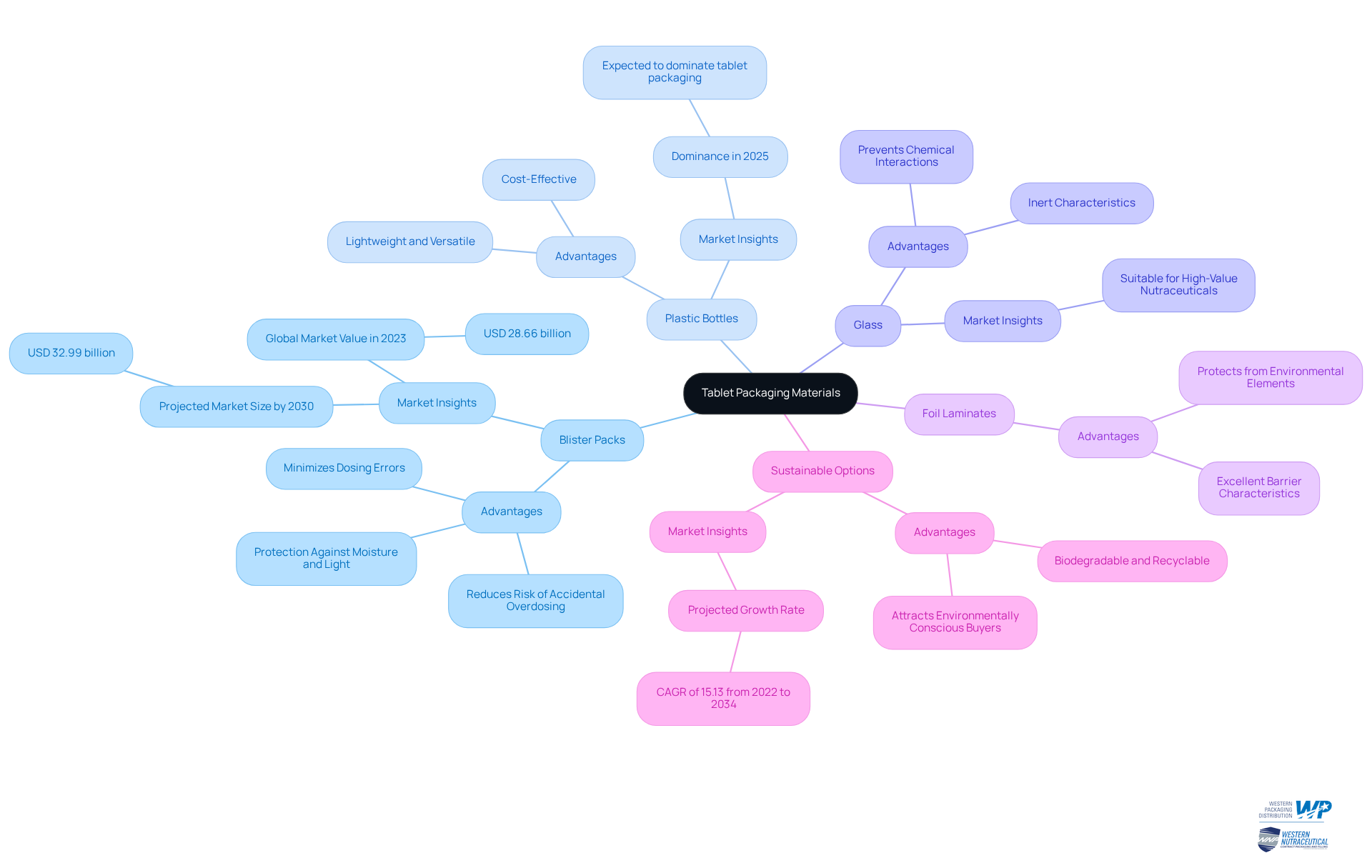
Select Appropriate Materials for Tablet Packaging
Selecting appropriate materials for tablet packaging requires a thorough evaluation of several options:
-
Blister Packs: Blister packs are increasingly favored for their exceptional protection against moisture and light, making them particularly suitable for sensitive tablets. They significantly reduce the risk of accidental overdosing, especially in children, and minimize dosing errors for daily medications. The global blister wrapping market is projected to reach USD 32.99 billion by 2030, reflecting the growing demand for such solutions in the nutraceutical sector. Furthermore, the pharmaceutical blister containers market size reached USD 23.31 billion in 2025, indicating robust growth in this area. As noted by Grand View Research, "Blister packs ensure the safety and integrity of medications, supplements, and medical devices during storage and transportation."
-
Plastic Bottles: Lightweight and versatile, plastic bottles are frequently utilized for tablet storage, holding a substantial market share. In 2025, tablet packaging is expected to be dominated by plastic bottles due to their convenience and cost-effectiveness. It is essential to ensure they are made from high-quality, food-grade materials to maintain item integrity.
-
Glass: Often favored for its inert characteristics, glass prevents chemical interactions with the contents. This makes it appropriate for high-value nutraceuticals, ensuring that the item remains stable and effective over time.
-
Foil Laminates: These substances provide excellent barrier characteristics, safeguarding tablets from environmental elements like moisture and oxygen, which can undermine product quality.
-
Sustainable Options: With growing consumer awareness, it is prudent to consider biodegradable or recyclable products to attract environmentally conscious buyers. The sustainable pharmaceutical container market is projected to grow at a CAGR of 15.13% from 2022 to 2034, indicating a strong shift towards eco-friendly practices. The introduction of biodegradable substances and the use of post-consumer recycled (PCR) plastics are becoming more common in the industry.
By thoughtfully choosing tablet packaging materials, manufacturers can enhance item stability and customer attraction while ensuring adherence to industry standards. For instance, effective use of blister packs has been shown to improve patient adherence and safety, making them a preferred choice in the nutraceutical industry.
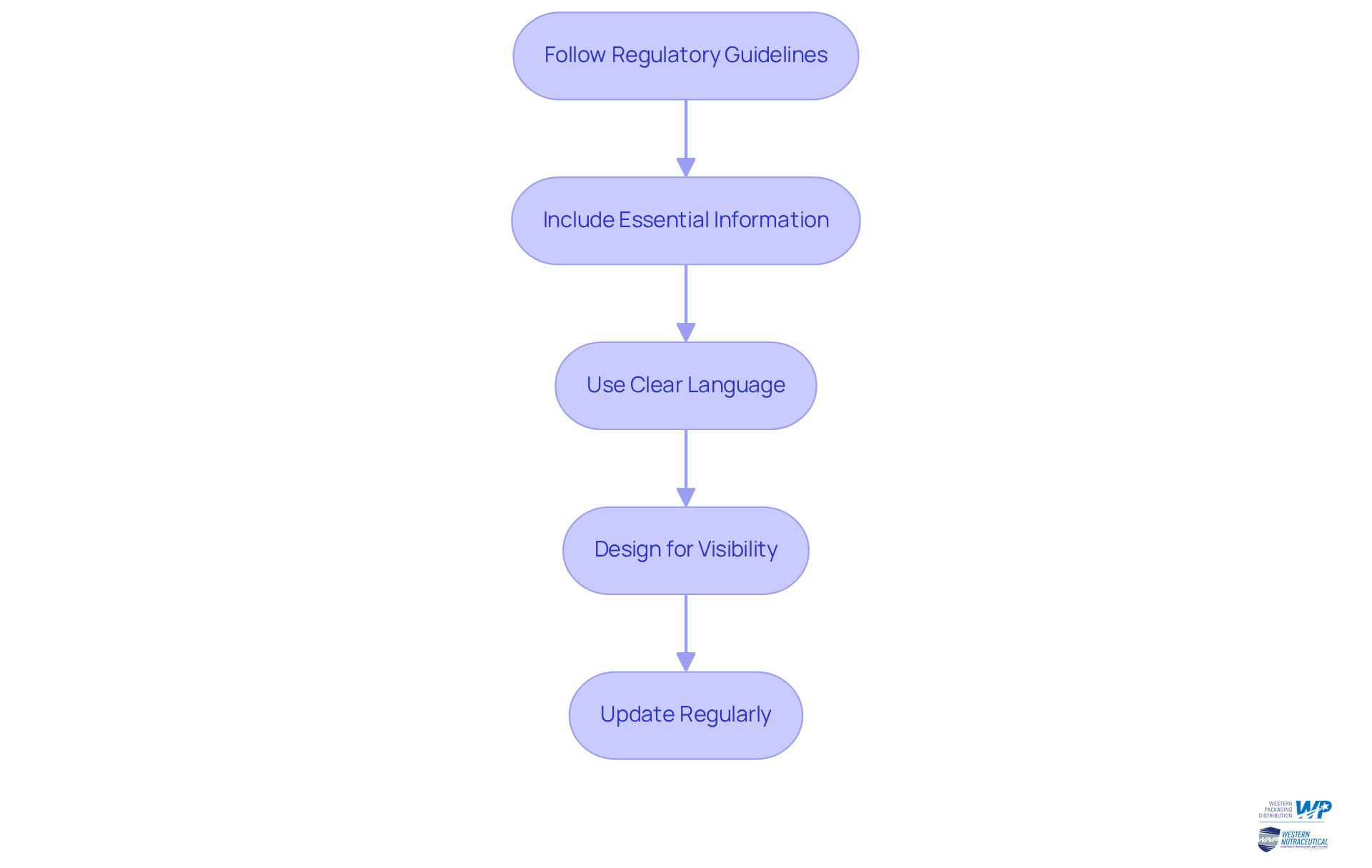
Adhere to Labeling Standards for Compliance and Clarity
To ensure compliance and clarity in labeling, manufacturers should:
- Follow Regulatory Guidelines: Familiarize yourself with the FDA's labeling requirements for dietary supplements, updated annually to reflect current standards and practices.
- Include Essential Information: Labels must clearly display the item name, ingredients, dosage instructions, and any relevant warnings or contraindications, as these elements are crucial for buyer safety and informed purchasing decisions.
- Use Clear Language: Avoid jargon and ensure that the language is straightforward and easily understandable to the typical buyer, enhancing the likelihood of purchase and trust in the product.
- Design for Visibility: Employ clear fonts and contrasting hues to enhance readability, especially for vital information like serving sizes and health claims, which can significantly affect buyer decisions. According to a study by the FDA, 70% of buyers reported that clear labeling influenced their purchasing decisions.
- Update Regularly: Stay informed about changes in regulations and evolving customer preferences to keep labels current and compliant, thereby maintaining transparency and trust. A notable example is Company X, which updated its labeling practices based on customer feedback and experienced a 30% rise in sales within six months.
By adhering to these labeling standards, manufacturers can significantly enhance transparency and cultivate greater trust among buyers, ultimately driving sales and brand loyalty. As industry expert Don Moxley states, "Clear labeling not only meets regulatory requirements but also builds a bridge of trust with consumers, leading to increased loyalty and sales.
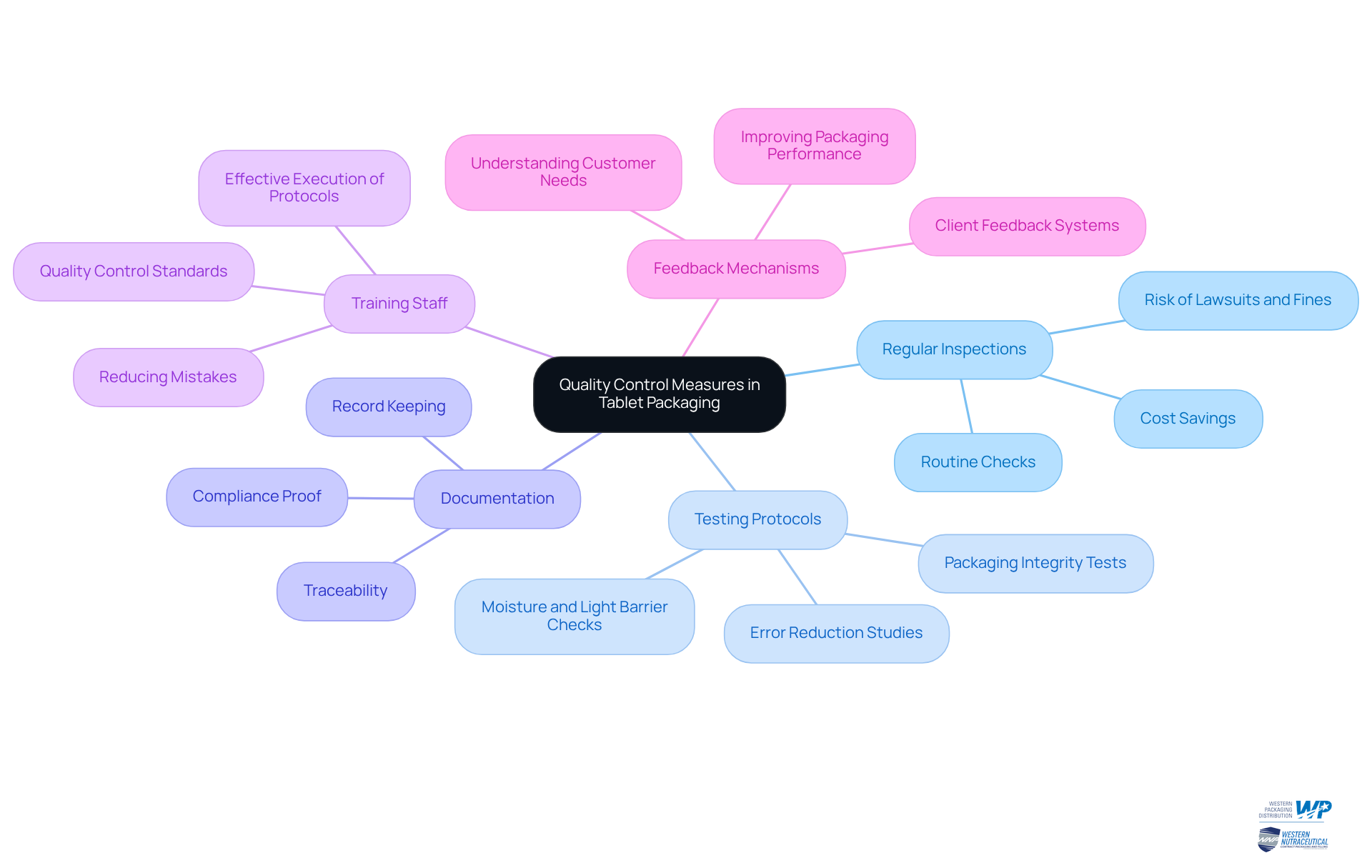
Implement Quality Control Measures in Tablet Packaging
Implementing robust quality control measures in tablet packaging is essential for ensuring product safety and compliance. Key practices include:
-
Regular Inspections: Conducting routine checks of materials and processes is vital for early identification of potential issues. Routine evaluations can avert expensive recalls and improve adherence to industry standards. Damaged containers can result in major customer problems and substantial penalties. Neglecting regular container inspections could put your business at significant risk of lawsuits and fines.
-
Testing Protocols: Establishing rigorous testing protocols for packaging integrity is crucial. This includes checks for moisture and light barriers, which are essential for maintaining product quality. Studies indicate that effective testing can significantly reduce the incidence of packaging-related errors, thereby safeguarding consumer health.
-
Documentation: Maintaining detailed records of quality control processes, including material specifications and inspection results, ensures traceability and compliance. Documentation is essential, as it offers proof of compliance with quality standards and can safeguard against legal problems stemming from product failures.
-
Training Staff: Ensuring that all personnel involved in the wrapping process are well-trained in quality control standards and practices is fundamental. An informed workforce can effectively execute inspection protocols, decreasing the chance of mistakes that could undermine quality.
-
Feedback Mechanisms: Implementing systems for gathering client feedback regarding packaging performance can inform future enhancements. Interacting with customers assists producers in comprehending their requirements and anticipations, ultimately improving item attractiveness and safety.
By prioritizing these quality control measures, manufacturers can significantly enhance product safety and consumer satisfaction while minimizing the risk of recalls or regulatory issues. Regular inspections, in particular, are a cornerstone of effective quality management, as they help identify and rectify potential problems before they escalate.
Conclusion
Effective tablet packaging is crucial for the success of nutraceutical products, ensuring that they meet consumer expectations while adhering to industry regulations. This packaging's significance extends beyond mere protection; it encompasses user convenience, dosage accuracy, and sustainability—imperatives for fostering brand loyalty and enhancing market appeal.
Key practices highlighted throughout this article include:
- Establishing essential packaging requirements
- Selecting appropriate materials
- Adhering to labeling standards
- Implementing quality control measures
Each of these components plays a vital role in ensuring that nutraceuticals remain safe, effective, and attractive to consumers. The insights provided underscore the necessity for manufacturers to stay informed about market trends and evolving regulations to maintain compliance and consumer trust.
Ultimately, the landscape of tablet packaging for nutraceuticals is evolving, with a clear shift towards sustainable practices and user-friendly designs. Manufacturers are encouraged to prioritize these aspects in their packaging strategies, not only to safeguard their products but also to resonate with the growing eco-conscious consumer base. By embracing these best practices, companies can enhance their market position and contribute to a healthier, more sustainable future in the nutraceutical industry.
Frequently Asked Questions
What are the essential packaging requirements for tablets?
The essential packaging requirements for tablets include item protection, dosage accuracy, user convenience, regulatory compliance, and sustainability.
How does packaging protect tablets?
Packaging must shield tablets from environmental factors such as moisture, light, and oxygen, which can compromise their integrity. Solutions like blister packs are designed to protect medications from these elements, ensuring their potency and safety.
Why is dosage accuracy important in tablet packaging?
Dosage accuracy is crucial as containers must facilitate precise dosing, especially for products requiring exact measurements. Packaging solutions like sachets and blister packs enhance dosing accuracy, making it easier for patients to administer the correct amount.
What role does user convenience play in tablet packaging?
User convenience is vital for customer satisfaction; the container should be easy to open and reseal. User-friendly designs enhance compliance with medication schedules, particularly for seniors who may find intricate containers challenging.
What regulations must manufacturers consider for tablet packaging?
Manufacturers must be knowledgeable about local and international regulations regarding container materials and labeling. Compliance with these regulations ensures safety and prevents legal complications.
How does sustainability impact tablet packaging?
Sustainability is increasingly important as consumers prefer eco-friendly options. Using recyclable or biodegradable materials can reduce environmental impact and appeal to eco-conscious buyers, influencing purchasing decisions.
How does Western Packaging address these packaging requirements?
Western Packaging focuses on innovative flexible solutions that enhance item protection, improve dosing accuracy, ensure user convenience, comply with regulations, and offer sustainable options for tablet packaging.




