Overview
Pharma containers are meticulously engineered to fulfill the stringent safety, functionality, and sustainability demands of the pharmaceutical industry, setting them apart from conventional packaging solutions. These containers not only offer superior protection and ensure regulatory compliance but also highlight a critical consideration: traditional packaging provides notable cost-effectiveness and versatility. Therefore, a thorough evaluation of each option is essential, based on the specific requirements of the product at hand.
Introduction
Pharmaceutical packaging is pivotal in ensuring the safety and efficacy of medications, and the landscape is evolving at an unprecedented pace. The emergence of specialized pharma containers, crafted to meet stringent regulatory standards, prompts a compelling comparison between these advanced solutions and traditional packaging methods.
As manufacturers grapple with the complexities of functionality, safety, and sustainability, a pressing question arises: which packaging approach truly strikes the optimal balance of protection, compliance, and environmental responsibility?
Delving into this comparison not only uncovers the strengths and weaknesses of each option but also highlights the critical considerations that will shape the future of pharmaceutical packaging.
Understanding Pharma Containers and Traditional Packaging
Pharma containers represent meticulously crafted solutions designed specifically for the unique demands of the drug industry. These containers—comprising vials, blister packs, and ampoules—are engineered to protect sensitive medications from environmental factors such as moisture and light, thereby ensuring their safety and efficacy.
In contrast, conventional containers encompass a broader array of materials and designs, including cardboard boxes, plastic bottles, and glass jars, which have been utilized across various industries for many years. While traditional packaging primarily emphasizes basic utility, pharma containers are distinctly focused on meeting rigorous safety and regulatory compliance standards.
This distinction is crucial, as the drug industry necessitates pharma containers that not only preserve product integrity but also comply with stringent industry regulations. As the market evolves, innovations in medicinal containers are increasingly addressing these requirements, with projections indicating market expansion to USD 356.7 billion by 2034, driven by advancements in technology and a heightened emphasis on sustainable practices.
Comparison Criteria: Functionality, Safety, and Sustainability
In the comparison of pharma containers and conventional wraps, three pivotal criteria emerge: functionality, safety, and sustainability.
- Functionality relates to the container's effectiveness in safeguarding the item while aiding its use. This includes considerations such as ease of opening and dosage accuracy, which are vital for user experience.
- Safety is essential, concentrating on the container's capacity to avert contamination and preserve the item's effectiveness during its shelf life. Advanced attributes such as tamper-evident seals and moisture barriers are crucial in pharma containers to maintain product integrity. Furthermore, the FDA mandates that medication container labels must include administration instructions, precise doses, usage cautions, and an exhaustive component list, underscoring the importance of safety compliance.
- Sustainability has grown in importance, reflecting the environmental effects of container materials and their lifecycle. The sustainable drug container market was valued at $71.6 billion in 2022 and is expected to reach $146.3 billion by 2027, indicating a significant move towards environmentally friendly practices. With 49% of U.S. consumers steering clear of items with excessive wrapping, the need for recyclable and biodegradable alternatives is increasing. This change is influenced not just by consumer preferences but also by regulatory pressures, as adherence to standards like ISO 15378 is essential for medical products. Packaging experts stress that incorporating sustainable methods into design can improve brand reputation while fulfilling regulatory standards.
Ultimately, the interplay of functionality, safety, and sustainability shapes the choices of pharma containers in the pharmaceutical industry, influencing purchasing decisions and compliance with stringent regulations. As the market evolves, manufacturers must prioritize these criteria to align with consumer expectations and regulatory standards.
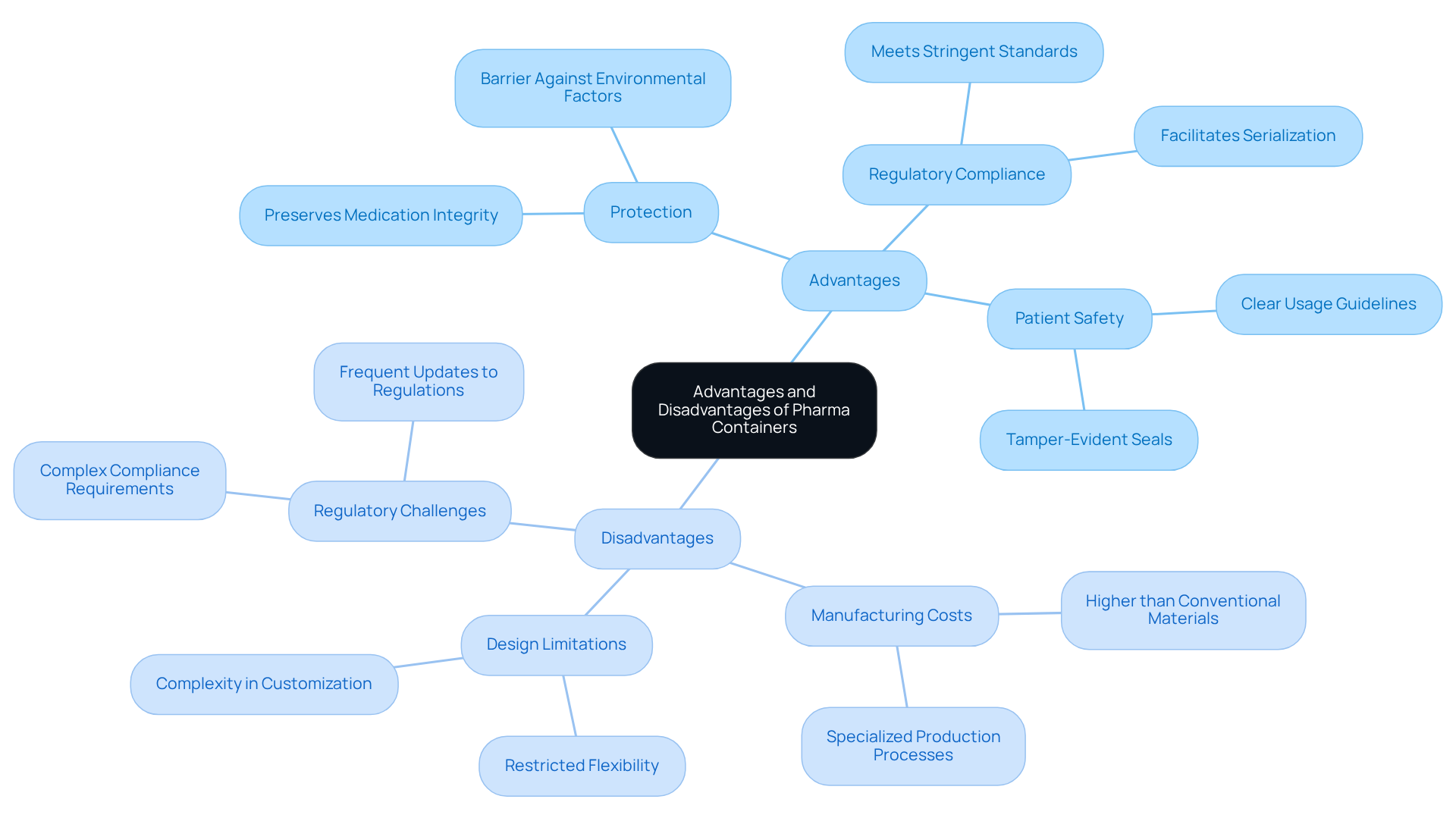
Advantages and Disadvantages of Pharma Containers
Pharma containers offer significant advantages, particularly enhanced protection against environmental factors, compliance with stringent regulatory standards, and improved patient safety through features like tamper-evident seals. These containers are meticulously designed to preserve the integrity of sensitive medications, a critical factor in ensuring their efficacy.
However, it is essential to acknowledge the disadvantages as well. The manufacturing costs of pharma containers may surpass those of conventional materials, and their specialized nature could restrict design and usage flexibility. Furthermore, the complexity of regulatory compliance presents challenges for manufacturers. Understanding these dynamics is crucial for making informed decisions in the packaging and logistics field.
Advantages and Disadvantages of Traditional Packaging
Traditional wrapping continues to be a preferred choice among manufacturers, primarily due to its cost-effectiveness and widespread availability. Its inherent versatility allows for a diverse range of designs and materials, accommodating various items across multiple industries. For instance, conventional wrapping can be easily customized, providing producers with the adaptability needed to meet specific branding and functional requirements. However, it is crucial to acknowledge that traditional containers may not offer the same level of protection as specialized pharma containers, particularly for sensitive products that demand stringent safety measures.
Moreover, the environmental impact of conventional materials cannot be overlooked. The dependence on non-biodegradable materials and the limited recyclability options contribute to rising concerns regarding sustainability. As the global market for containers evolves, manufacturers face increasing pressure to implement eco-friendly practices. In fact, the sustainable plastic wrapping market is projected to reach nearly 157.66 billion USD by 2032, indicating a significant shift towards environmentally responsible solutions. This transition underscores the necessity for manufacturers to consider not only the direct costs of containers but also indirect expenses such as storage and transportation, which can significantly influence total expenditures.
Industry specialists emphasize the importance of balancing cost with environmental considerations. As Adam Peek noted, "It’s time to enhance your item wrapping and achieve cost reductions while improving your customers’ experience." This statement highlights the need for manufacturers to meticulously evaluate their pharma containers options, weighing the benefits of conventional wrapping against the potential long-term impacts on both profitability and the environment. Furthermore, the challenges associated with recycling plastics and the growing skepticism surrounding plastic products necessitate a more thoughtful approach to container selection.
Conclusion: Choosing the Right Packaging Solution for Your Needs
Selecting the appropriate container solution is pivotal and relies on several critical factors, including the characteristics of the item, regulatory standards, and market needs. For pharmaceutical items that necessitate stringent safety and efficacy standards, the optimal choice is pharma containers, thanks to their specialized design and compliance features. Conversely, conventional wrapping may be more suitable for items that do not undergo the same degree of scrutiny or for producers seeking economical options. Ultimately, a thorough understanding of the advantages and disadvantages of each type of packaging empowers businesses to make informed decisions that enhance product integrity and bolster market presence.
Conclusion
The choice between pharma containers and traditional packaging stands as a pivotal consideration for the pharmaceutical industry, highlighting the necessity for tailored solutions that meet specific product requirements. Pharma containers are meticulously designed with a focus on safety, compliance, and functionality, ensuring that sensitive medications retain their integrity throughout their lifecycle. In contrast, traditional packaging provides versatility and cost-effectiveness, though it may lack the specialized features essential for safeguarding pharmaceuticals.
Key insights from this comparison indicate that while pharma containers excel in protecting medications from environmental factors and meeting regulatory standards, traditional packaging remains a viable option for less sensitive products. The increasing emphasis on sustainability complicates this decision further, as both types of packaging are subjected to growing scrutiny concerning their environmental impact. By understanding the advantages and disadvantages of each option, manufacturers are empowered to make informed choices that align with market demands and regulatory frameworks.
Ultimately, the future of pharmaceutical packaging depends on a balanced approach that prioritizes safety, functionality, and sustainability. As the industry continues to evolve, manufacturers must remain vigilant in assessing their packaging strategies, ensuring they not only comply with current standards but also anticipate future trends. Embracing innovative packaging solutions will be crucial for enhancing product integrity and fostering consumer trust in an increasingly competitive marketplace.
Frequently Asked Questions
What are pharma containers and how do they differ from traditional packaging?
Pharma containers are specifically designed solutions for the drug industry, including vials, blister packs, and ampoules, which protect medications from environmental factors. In contrast, traditional packaging includes a wider range of materials like cardboard boxes and plastic bottles, focusing on basic utility rather than the rigorous safety and regulatory compliance required in the pharmaceutical sector.
Why is the distinction between pharma containers and traditional packaging important?
The distinction is important because the drug industry requires containers that not only preserve product integrity but also comply with strict industry regulations. Pharma containers are engineered to meet these demands, ensuring the safety and efficacy of medications.
What are the key criteria for comparing pharma containers and conventional packaging?
The key criteria for comparison are functionality, safety, and sustainability. Functionality involves the container's effectiveness in protecting the item and aiding its use. Safety focuses on preventing contamination and maintaining the item's effectiveness. Sustainability addresses the environmental impact of container materials and their lifecycle.
How does functionality impact the design of pharma containers?
Functionality impacts the design by ensuring that containers are effective in safeguarding medications while also being user-friendly, which includes ease of opening and dosage accuracy.
What safety features are important in pharma containers?
Important safety features include tamper-evident seals and moisture barriers, which help maintain product integrity. Additionally, FDA regulations require medication container labels to include administration instructions, precise doses, usage cautions, and a comprehensive component list.
What trends are influencing sustainability in pharma containers?
Sustainability is increasingly important due to the environmental effects of container materials and consumer preferences for recyclable and biodegradable options. The sustainable drug container market is projected to grow significantly, driven by regulatory pressures and consumer demand for environmentally friendly practices.
What is the projected market growth for pharma containers?
The market for pharma containers is projected to expand to USD 356.7 billion by 2034, driven by advancements in technology and a greater emphasis on sustainable practices.
How do the criteria of functionality, safety, and sustainability affect purchasing decisions in the pharmaceutical industry?
These criteria influence purchasing decisions by ensuring that pharma containers meet consumer expectations and comply with stringent regulations. Manufacturers must prioritize these aspects to remain competitive and uphold brand reputation.




