Overview
This article delves into the realm of pharmaceutical packaging, articulating its critical purpose, chronicling its evolution, and delineating its fundamental characteristics. It underscores that effective pharmaceutical packaging is vital for safeguarding medication safety and efficacy. Furthermore, it highlights the necessity for packaging to adapt to regulatory requirements and consumer preferences. This adaptability is evidenced by the increasing market demand for innovative and compliant packaging solutions, which reflect a commitment to quality and reliability in the industry.
Introduction
The realm of pharmaceutical packaging, often underestimated, is vital for safeguarding the safety and efficacy of medications. As the industry advances, the importance of innovative packaging solutions becomes increasingly evident, with technology and sustainability driving the future of drug containment. Yet, amidst escalating regulatory demands and consumer expectations, manufacturers face the challenge of balancing compliance with creativity to enhance both product integrity and brand trust. By examining the definition, evolution, and key characteristics of pharmaceutical packaging, we uncover not only its essential functions but also the hurdles that lie ahead in this dynamic field.
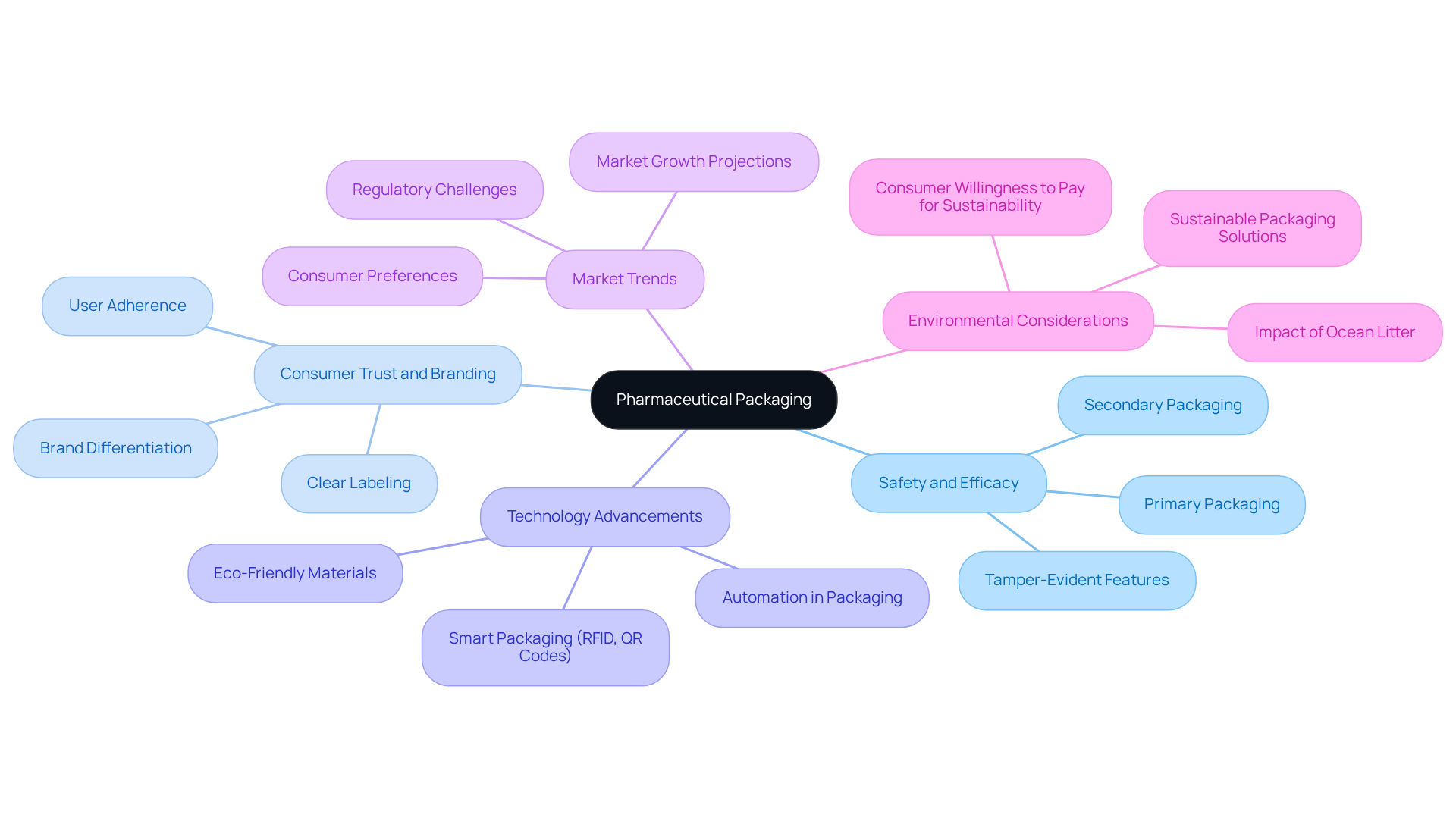
Define Pharmaceutical Packaging: Purpose and Significance
The methods used in pharma packaging for the containment of medications involve enclosing and protecting medical items for distribution, storage, sale, and use. Its primary objective is to ensure the safety, efficacy, and quality of medications, including the considerations of pharma packaging, while delivering critical information to consumers and healthcare professionals. Efficient pharma packaging safeguards items from contamination and deterioration, significantly improving user adherence through clear labeling and comprehensive instructions. Beyond simple containment, pharma packaging plays a vital role in branding, market differentiation, and building consumer trust—especially in the nutraceuticals sector, where preserving item integrity is essential.
At Western Packaging, we offer customized flexible wrapping solutions that seamlessly integrate with our advanced filling services and comprehensive 3PL solutions. Our services streamline your production process and enhance supply chain effectiveness, boosting product attractiveness and brand awareness through innovative design solutions. Recent advancements in container technology, including smart containers utilizing RFID tags and QR codes for real-time monitoring, are directly linked to our offerings, further enhancing safety and compliance. The worldwide drug container market was valued at USD 565.23 billion in 2023 and is projected to expand at a CAGR of 15.40% from 2025 to 2034, underscoring the increasing importance of innovative and eco-friendly containers. As the industry evolves, the focus on these approaches intensifies, reflecting the changing requirements of consumers and regulatory standards, with 43% of consumers considering environmental impact a significant factor in their purchasing decisions.
Trace the Evolution of Pharmaceutical Packaging: Historical Context
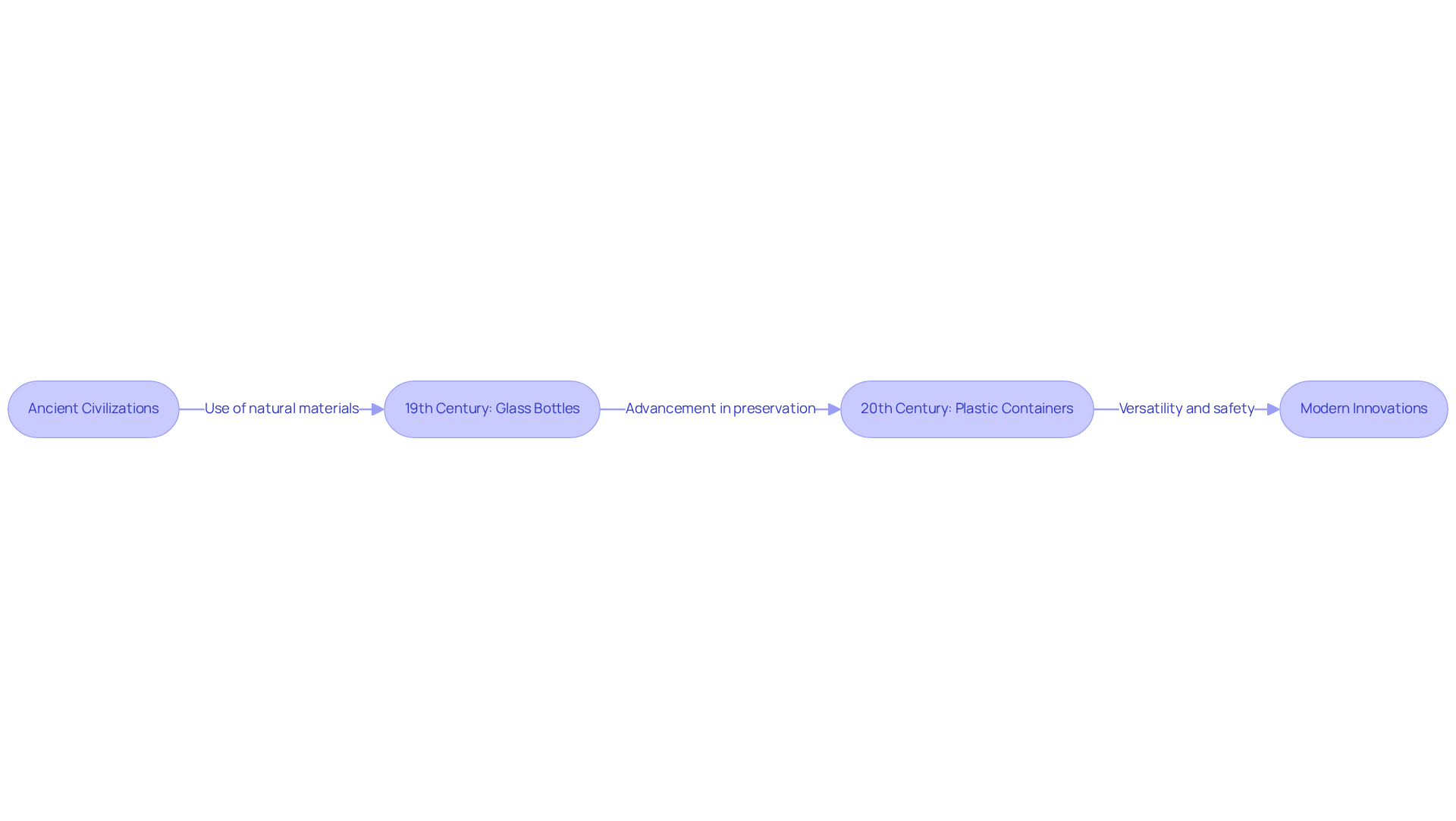
The evolution of medicine containers dates back to ancient civilizations, where natural materials such as leaves and animal hides were employed to contain healing substances. As the pharmaceutical sector expanded, the complexity of pharma packaging solutions also increased. The introduction of glass bottles in the 19th century represented a significant advancement, enabling better preservation of liquid medications. The 20th century heralded the rise of plastic containers, offering versatility and cost-effectiveness. Today, innovations like child-resistant closures, tamper-evident seals, and intelligent container technologies underscore the industry's dedication to safety and consumer protection.
Industry reports indicate that the drug container market is projected to grow at a compound annual growth rate (CAGR) of 16.1% during the forecast period, with a market value expected to reach USD 1589.54 billion by 2030. This historical perspective highlights the ongoing need for container solutions in pharma packaging that adapt to evolving market demands and regulatory standards, particularly in light of the FDA's stringent guidelines for pharmaceutical labels, which necessitate the inclusion of administration instructions and precise dosages.
Moreover, the pharma packaging industry faces challenges such as rising regulatory compliance costs, which exceed $10 billion annually, further emphasizing the importance of innovative and compliant container solutions.
Explore Types of Pharmaceutical Packaging: Functions and Characteristics
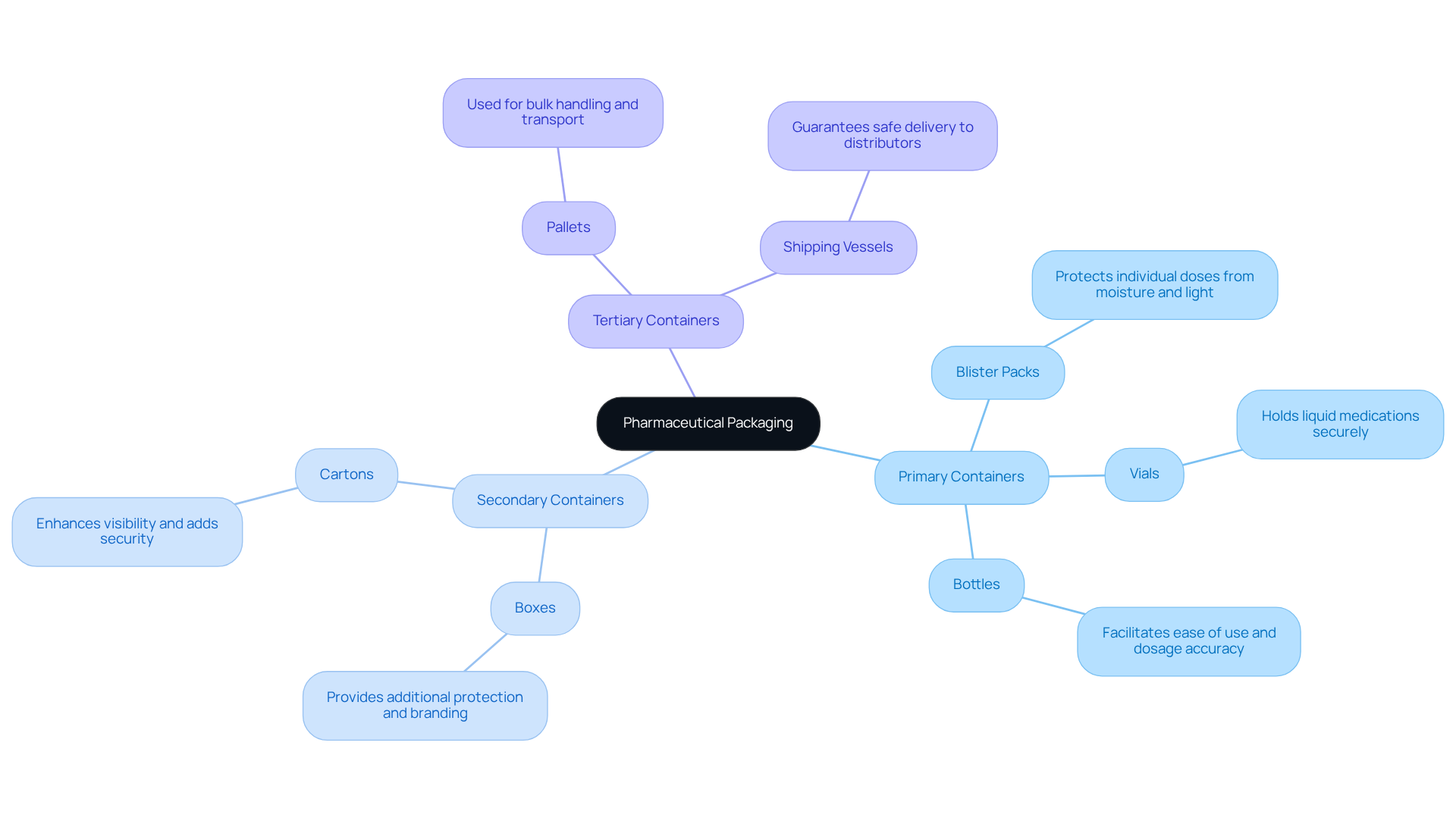
Pharmaceutical containers represent a broad spectrum of types, each meticulously crafted to fulfill specific roles within the industry. Primary containers, such as blister packs, vials, and bottles, directly hold the product and are critical for preserving medication integrity. Blister packs protect individual doses from moisture and light, while bottles facilitate ease of use and dosage accuracy.
Secondary containers, including boxes and cartons, provide additional protection and branding, enhancing visibility and adding extra layers of security during transportation. Tertiary containers, utilized for bulk handling and transport, encompass pallets and shipping vessels that guarantee safe delivery to distributors and retailers.
The selection of container types is influenced by various factors, including item stability, shelf life, and regulatory compliance. For instance, the choice between primary and secondary containers can significantly impact the product's market effectiveness.
As the pharmaceutical landscape evolves, trends such as sustainability and intelligent container technologies are becoming increasingly important in driving innovation in pharma packaging solutions. This dynamic environment necessitates that manufacturers remain vigilant in selecting the most suitable container options to meet both regulatory standards and consumer expectations.
Understand Regulatory Compliance in Pharmaceutical Packaging: Safety Standards and Guidelines
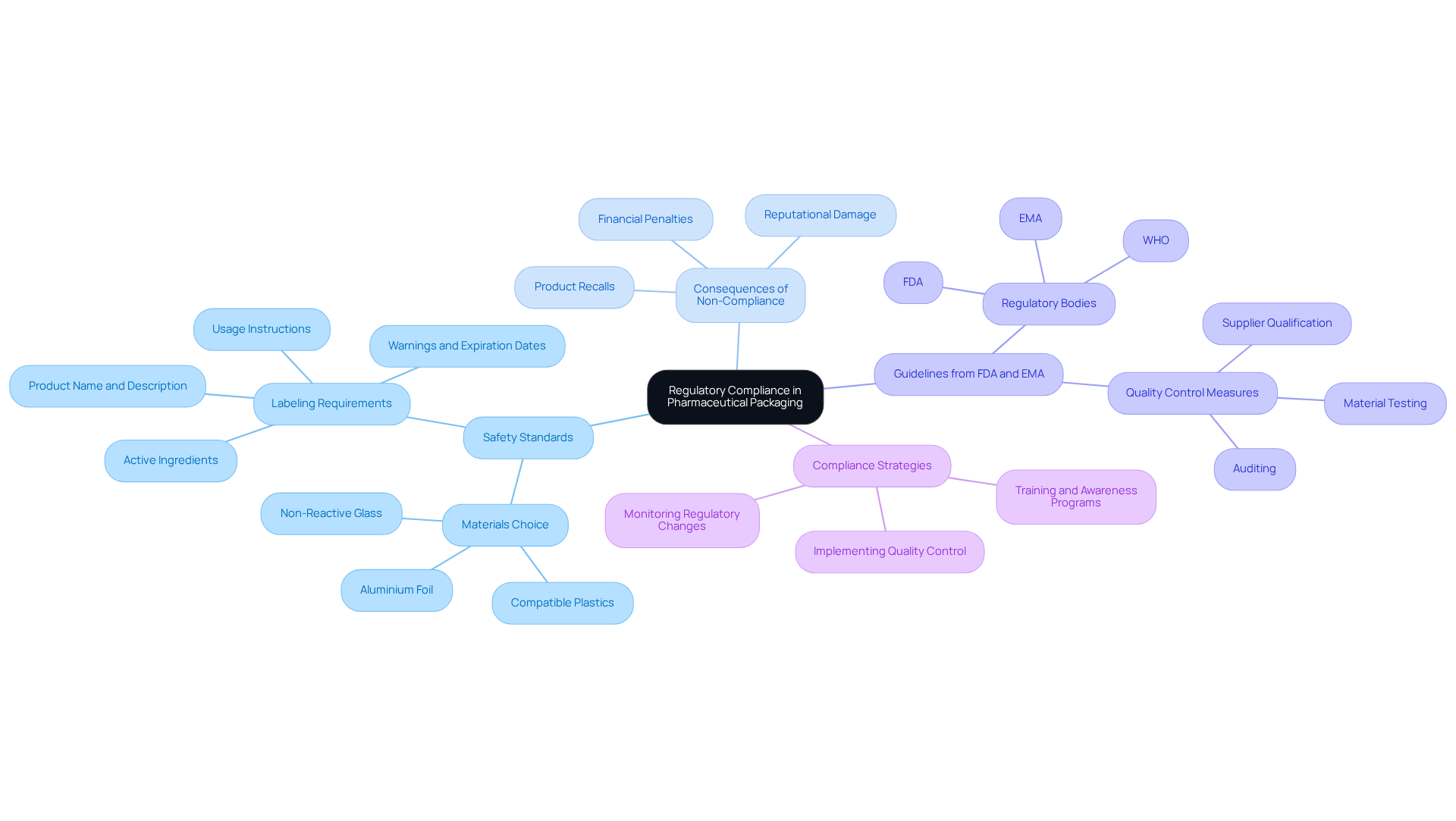
Regulatory compliance in pharmaceutical containers is governed by stringent safety standards and guidelines established by organizations such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). These regulations dictate various aspects of packaging, including the choice of materials, labeling requirements, and the incorporation of child-resistant features. Adherence is crucial not only for safeguarding the item but also for offering clear details about usage, dosage, and possible side effects. Non-compliance can lead to severe repercussions, including financial penalties, product recalls, and reputational damage.
For instance, an examination of FDA drug recall information from 2012 to 2023 reveals an average of 330 drug recalls initiated each year, with a significant portion linked to container issues. Additionally, from 2019 to 2023, the FDA increased the issuance of warning letters from 2.98 to 4.27 per 100 inspections, representing a 43% increase, which underscores the heightened scrutiny on compliance. A notable case involved a pharmaceutical firm recalling multiple batches of medication due to container problems that could have led to contamination.
Consequently, manufacturers must remain vigilant about evolving regulations and integrate compliance considerations into their packaging strategies. This includes implementing quality control measures such as supplier qualification and material testing to ensure consumer safety and maintain market credibility. By adhering to these guidelines, companies can enhance their operational efficiency and protect their brand integrity.
Conclusion
Pharmaceutical packaging is a critical component in ensuring the integrity, safety, and effectiveness of medications. It encompasses a range of functions, from protecting products against contamination to providing essential information to consumers and healthcare professionals. As the industry continues to evolve, the significance of innovative packaging solutions—including environmentally friendly options and intelligent technologies—has become increasingly evident.
The historical progression of pharmaceutical packaging reveals a journey from ancient materials to modern advancements such as child-resistant closures and smart containers. Regulatory compliance is paramount, with stringent guidelines set by organizations like the FDA and EMA. These regulations not only safeguard consumer health but also influence manufacturers' operational strategies and market credibility.
In light of these insights, it is crucial for stakeholders in the pharmaceutical industry to prioritize effective packaging solutions that meet both consumer expectations and regulatory requirements. Embracing innovation in packaging enhances product safety and efficacy while building consumer trust and brand loyalty. By recognizing the vital role of pharmaceutical packaging, the industry can continue to advance, ensuring that medications are delivered safely and effectively to those in need.
Frequently Asked Questions
What is the primary purpose of pharmaceutical packaging?
The primary purpose of pharmaceutical packaging is to enclose and protect medications for distribution, storage, sale, and use, ensuring the safety, efficacy, and quality of the products.
How does pharmaceutical packaging contribute to user adherence?
Pharmaceutical packaging improves user adherence by providing clear labeling and comprehensive instructions, which help consumers understand how to use the medications correctly.
What role does pharmaceutical packaging play in branding and consumer trust?
Pharmaceutical packaging plays a vital role in branding, market differentiation, and building consumer trust, particularly in the nutraceuticals sector, where preserving item integrity is crucial.
What innovative solutions does Western Packaging offer in pharmaceutical packaging?
Western Packaging offers customized flexible wrapping solutions that integrate with advanced filling services and comprehensive 3PL solutions, enhancing production processes and supply chain effectiveness.
What advancements in container technology are mentioned in the article?
Recent advancements include smart containers that utilize RFID tags and QR codes for real-time monitoring, which enhance safety and compliance.
What was the value of the worldwide drug container market in 2023, and what is its projected growth?
The worldwide drug container market was valued at USD 565.23 billion in 2023 and is projected to expand at a CAGR of 15.40% from 2025 to 2034.
What factors are influencing the focus on innovative and eco-friendly containers in the pharmaceutical industry?
The focus on innovative and eco-friendly containers is influenced by changing consumer requirements and regulatory standards, with 43% of consumers considering environmental impact a significant factor in their purchasing decisions.




