Overview
Selecting a contract manufacturer for nutritional supplements necessitates meticulous consideration of:
- Experience
- Certifications
- Production capabilities
- Quality control processes
This diligence is crucial to ensure compliance and maintain product integrity. A structured approach is essential; thorough research and clear communication are vital for establishing successful partnerships that can adapt to market demands and bolster brand reputation.
Introduction
Selecting the right contract manufacturer for nutritional supplements is a pivotal decision that can profoundly influence a brand's success. As demand for high-quality dietary products escalates, comprehending the intricacies of this outsourcing process becomes essential for companies aiming to excel in a competitive landscape.
The challenge, however, resides in navigating the multitude of options available and ensuring compliance with industry standards while safeguarding product integrity. Brands must prioritize key factors to establish a successful partnership that not only fulfills their production requirements but also amplifies their market presence.
Understand Contract Manufacturing for Nutritional Supplements
involves outsourcing the creation of dietary items to specialized entities. This strategy allows brands to leverage their expertise in producing high-quality supplements while adhering to stringent industry regulations. The approach offers several key benefits:
- Significant cost reductions
- Access to advanced equipment
- Expedited time-to-market
Industry leaders emphasize the importance of collaborating with producers that have a verified history, as this can lead to enhanced quality and compliance. Oskar highlights that transparency and robust communication are essential for successful partnerships, enabling brands to concentrate on their core competencies while ensuring product integrity.
Statistics indicate that U.S.-based production companies operate under rigorous regulatory supervision, which is vital for maintaining high standards in the nutraceutical sector. This oversight not only helps brands avoid compliance issues but also builds consumer trust. Additionally, successful manufacturing collaborations can yield tailored formulations that cater to specific consumer preferences, such as gluten-free or organic options, while securing higher-quality raw materials with certifications like USDA Organic and Non-GMO Project Verified.
The advantages of outsourcing production to a contract manufacturer of nutritional supplements extend beyond operational efficiencies; they encompass the capability to scale production in response to fluctuating market demands. As industry experts underscore, those who adapt to evolving consumer expectations and capitalize on new market opportunities will be well-positioned for success. In this competitive landscape, cooperation between brands and production partners is crucial, ensuring that both parties strive toward a shared objective of delivering that meet consumer demands.
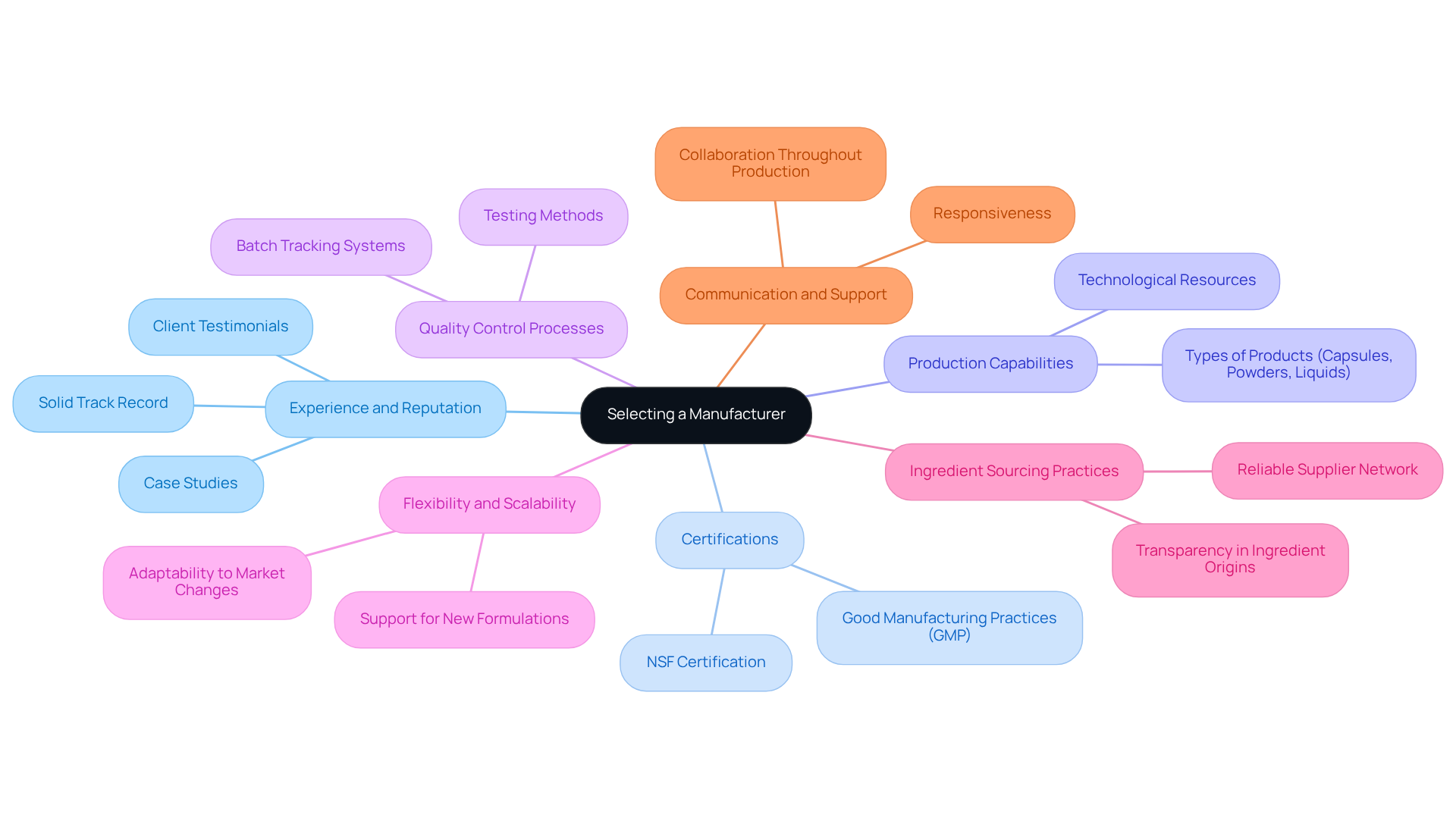
Evaluate Key Criteria for Selecting a Manufacturer
When selecting a contract manufacturer for nutritional supplements, several key criteria should guide your decision-making process:
- Experience and Reputation: Prioritize producers with a solid track record in the nutraceutical sector. Investigate their history, client testimonials, and case studies to gauge their reliability and success. As Evelyn Reinson, Director of Brand & Consumer Engagement, emphasizes, "In the fiercely competitive and rapidly expanding nutraceutical market, the selection of a contract producer can determine the trajectory of a supplement company."
- Certifications: Confirm that the producer possesses essential certifications, such as Good Manufacturing Practices (GMP) and NSF certification. These credentials are vital indicators of their commitment to quality and compliance with industry standards. The importance of GMP certification cannot be overstated, as it ensures adherence to safety and quality protocols.
- Production Capabilities: Assess the producer's capacity to create your particular types, whether capsules, powders, or liquids. Evaluate their equipment and technological resources to ensure they can meet your production needs. The worldwide market for contract manufacturer nutritional supplements services was valued at USD 133.34 billion in 2022 and is expected to expand at a compound annual growth rate of 12.04% from 2023 to 2030, emphasizing the demand for skilled producers.
- Quality Control Processes: Inquire about their quality assurance protocols, including testing methods and batch tracking systems. A reputable producer will have strong quality control measures to ensure consistency and safety of items. This is crucial in maintaining product integrity and meeting regulatory standards.
- Flexibility and Scalability: Select a producer that can adjust to your changing needs, whether increasing production or accommodating new formulations. This flexibility is crucial for responding to market demands effectively.
- Ingredient Sourcing Practices: Evaluate the producer's ingredient sourcing methods and their network of reliable suppliers. Transparency regarding ingredient origins and testing procedures is essential for product integrity.
- Communication and Support: Assess their responsiveness and willingness to collaborate throughout the production process. Effective communication is essential for a successful partnership, ensuring that your needs are met promptly and efficiently.
By concentrating on these criteria, you can choose a production partner that not only fulfills your production requirements but also boosts your brand's reputation and market presence in the competitive nutraceutical landscape.
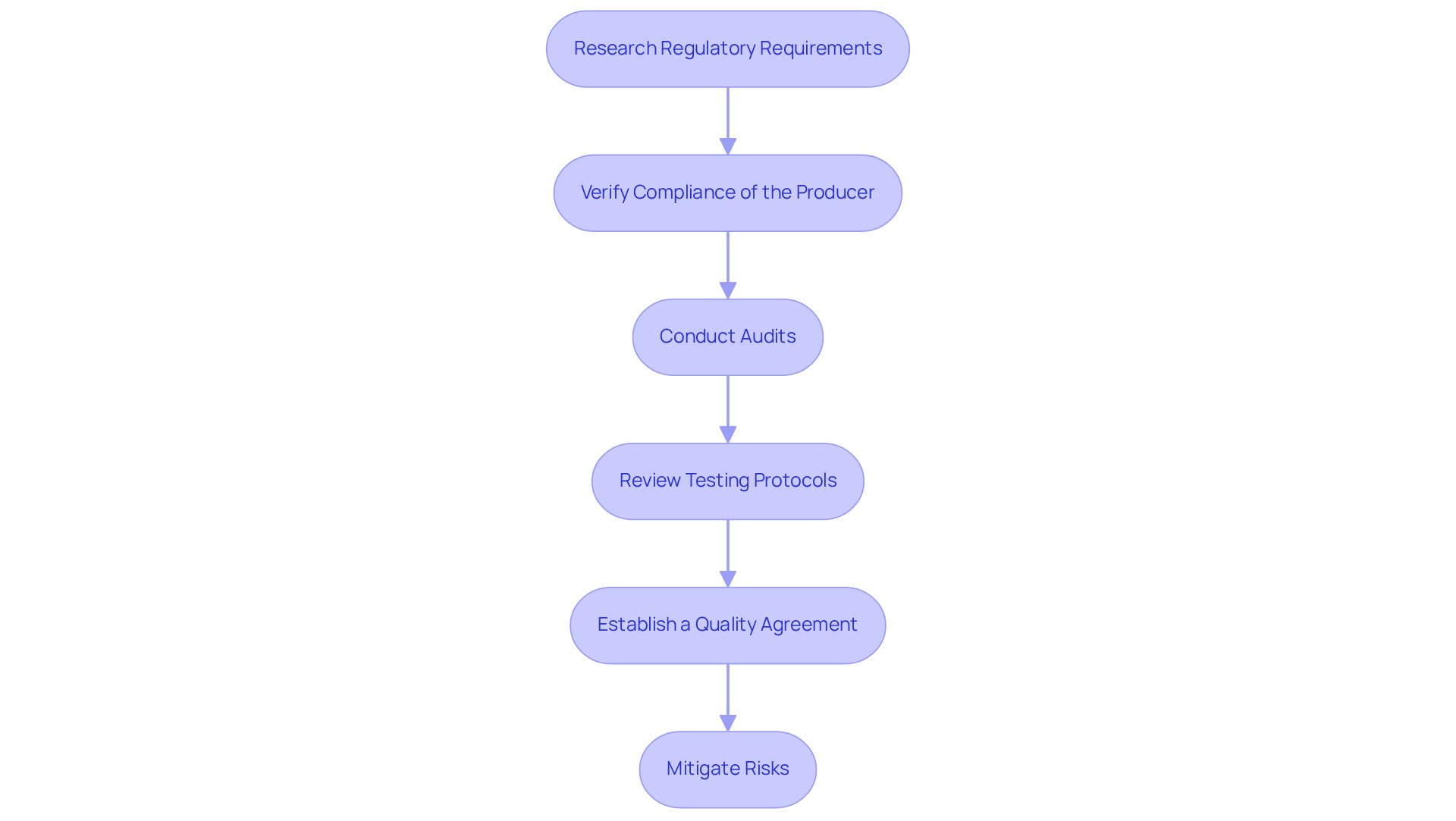
Ensure Regulatory Compliance and Quality Control
To ensure regulatory compliance and uphold high-quality standards when selecting a contract manufacturer for nutritional supplements, consider the following steps:
- Research Regulatory Requirements: Begin by understanding the regulations governing dietary supplements, particularly the Dietary Supplement Health and Education Act (DSHEA) and the guidelines established by the FDA. Familiarity with these regulations is crucial for compliance.
- Verify Compliance of the Producer: Request comprehensive documentation demonstrating the producer's adherence to (cGMP) and other pertinent regulations. Compliance rates within the nutraceutical sector serve as essential indicators of a producer's reliability. Notably, recent statistics reveal that only 60% of producers consistently meet cGMP standards, underscoring the importance of thorough verification.
- Conduct Audits: Whenever feasible, perform on-site audits of the manufacturing facility. This allows for a direct evaluation of their quality control procedures and compliance with safety standards, which is vital for ensuring integrity. Successful audits have shown that producers who maintain strict quality control protocols significantly reduce the risk of non-compliance.
- Review Testing Protocols: Confirm that the manufacturer implements rigorous testing procedures for both raw materials and finished products. This ensures that the potency and purity of the supplements meet established standards, safeguarding consumer health. As industry leader Bryan Losier emphasizes, "Strict adherence to testing protocols is essential for maintaining quality and consumer trust."
- Establish a Quality Agreement: Create a detailed quality agreement that clearly delineates the responsibilities of both parties regarding quality control, testing, and compliance. This formalizes expectations and fosters accountability in the manufacturing process.
By adhering to these steps, producers can significantly mitigate risks associated with non-compliance, which can lead to costly recalls and damage to brand reputation. The importance of cGMP compliance in contract manufacturer nutritional supplements cannot be overstated, as it serves as a foundation for ensuring product safety and efficacy.
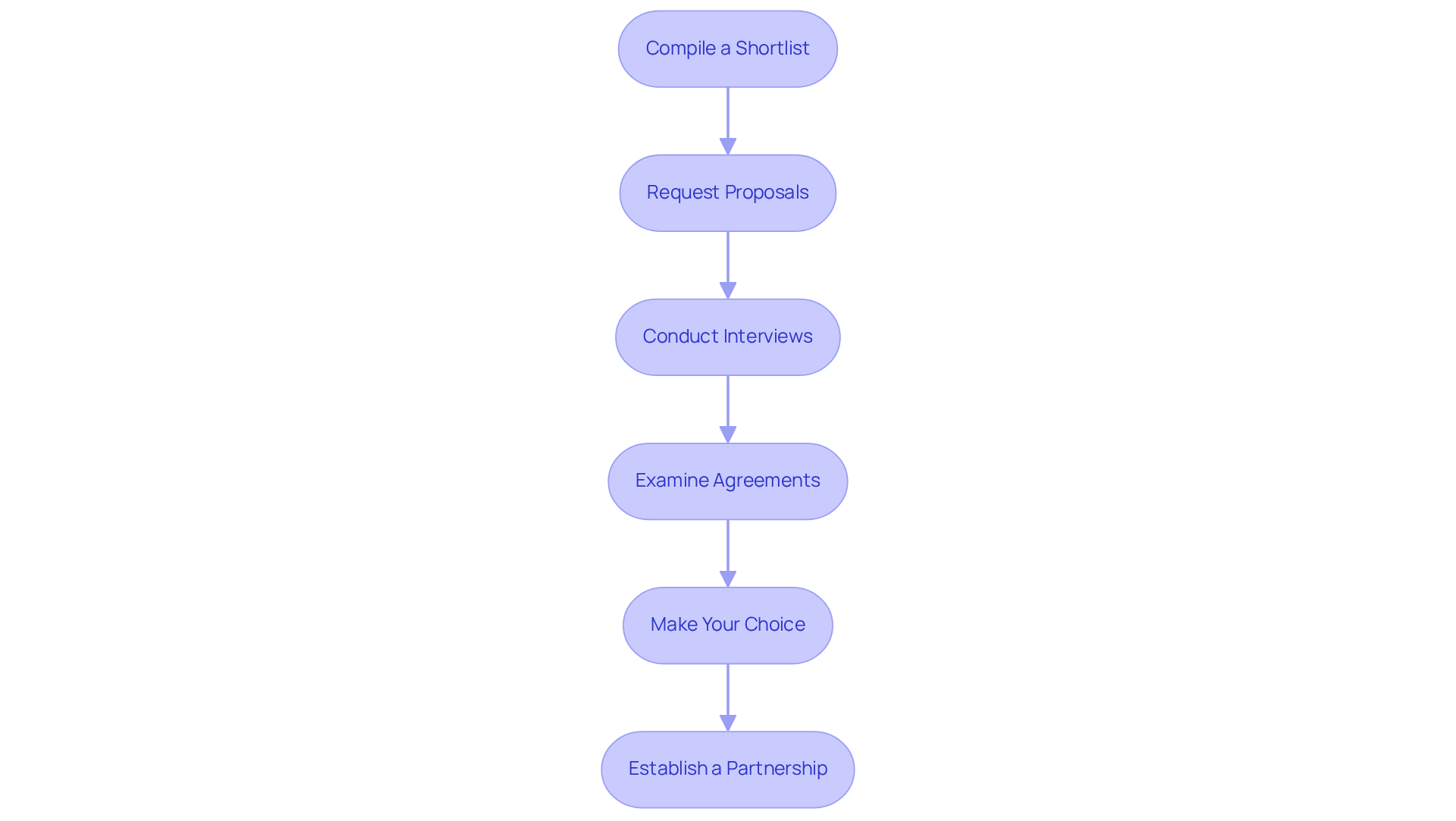
Follow a Step-by-Step Process to Finalize Your Choice
To finalize your choice of a contract manufacturer for nutritional supplements, follow these essential steps:
- Compile a Shortlist: Begin by identifying potential producers that align with your specific requirements. Focus on those with a proven track record in the nutraceutical industry, ensuring they meet necessary certifications and quality standards, including compliance with FDA regulations.
- Request Proposals: Reach out to your shortlisted candidates and request . These should detail their services, pricing structures, lead times, and any minimum order quantities (MOQs) they may impose, which can be as low as 500 or 1,000 units for startups. Be cautious of vague quotes and surprise fees related to label setup, R&D, testing, or packaging materials.
- Conduct Interviews: Schedule interviews with the top candidates to delve deeper into their capabilities. This is an opportunity to discuss your project specifics and gauge their responsiveness and compatibility with your brand ethos.
- Examine Agreements: Analyze the agreements supplied by each producer. Pay close attention to terms regarding pricing, timelines, quality assurance protocols, and any clauses that may affect your partnership.
- Make Your Choice: After comprehensive assessments, interviews, and agreement reviews, choose the supplier that most effectively fulfills your operational requirements and strategic objectives. Consider the potential for increased production volumes without worrying about capacity limitations, which can be a significant benefit of collaborating with an external producer.
- Establish a Partnership: Once you’ve made your selection, focus on building a strong partnership. A reliable producer should be a long-term ally, not merely a one-time supplier. Maintain open lines of communication and set clear expectations to ensure a smooth production process.
By following this structured approach, you can enhance your chances of establishing a successful partnership with a contract manufacturer for nutritional supplements, ultimately leading to high-quality products that resonate with your target market.
Conclusion
Selecting the right contract manufacturer for nutritional supplements is a pivotal decision that can profoundly impact a brand's success. By outsourcing production to a specialized entity, companies can realize significant cost reductions, gain access to advanced technology, and enhance compliance with industry regulations. This strategic partnership not only enables brands to concentrate on their core competencies but also guarantees that the products meet the high standards consumers expect.
Key considerations for choosing a manufacturer encompass:
- Their experience and reputation
- Necessary certifications
- Production capabilities
- Quality control processes
Each of these factors is essential in establishing a reliable partnership that can adapt to market demands and consumer preferences. Ensuring regulatory compliance and maintaining quality control are critical steps that protect both the brand's integrity and consumer trust.
Ultimately, the process of selecting a contract manufacturer should be approached with diligence and strategic foresight. By adhering to a structured method, brands can cultivate partnerships that enhance their market presence and deliver high-quality nutritional supplements. In a competitive landscape, investing time and resources in this selection process is not merely beneficial; it is vital for sustaining growth and addressing the evolving needs of consumers.
Frequently Asked Questions
What is contract manufacturing for nutritional supplements?
Contract manufacturing for nutritional supplements involves outsourcing the creation of dietary items to specialized entities, allowing brands to focus on their core competencies while ensuring high-quality production.
What are the benefits of using a contract manufacturer for nutritional supplements?
The benefits include significant cost reductions, access to advanced equipment, and expedited time-to-market for products.
Why is it important to collaborate with producers that have a verified history?
Collaborating with producers that have a verified history enhances quality and compliance, which is crucial for maintaining industry standards.
How does transparency and communication affect partnerships in contract manufacturing?
Transparency and robust communication are essential for successful partnerships, enabling brands to maintain product integrity while focusing on their core business activities.
What regulatory environment do U.S.-based production companies operate under?
U.S.-based production companies operate under rigorous regulatory supervision, which is vital for maintaining high standards in the nutraceutical sector and helps brands avoid compliance issues.
How can successful manufacturing collaborations benefit product offerings?
Successful collaborations can yield tailored formulations that cater to specific consumer preferences, such as gluten-free or organic options, while also ensuring higher-quality raw materials with certifications like USDA Organic and Non-GMO Project Verified.
How does outsourcing production help brands respond to market demands?
Outsourcing production allows brands to scale production in response to fluctuating market demands, helping them adapt to evolving consumer expectations and capitalize on new market opportunities.
What is the significance of cooperation between brands and production partners?
Cooperation is crucial as it ensures both parties work toward a shared objective of delivering top-quality nutritional products that meet consumer demands in a competitive landscape.




