Overview
The article delves into the significance and evolution of pharmaceutical terminology within the industry, underscoring its critical role in enhancing communication and ensuring safety in healthcare practices. It articulates the historical development of terminology, examines key components such as drug classifications and regulatory terms, and highlights the profound impact that precise language has on medication development and patient care.
Understanding this evolution is essential for professionals in the field, as it not only fosters better communication but also underpins the safety protocols that protect patients. The mastery of pharmaceutical terminology is not merely an academic exercise; it is a fundamental aspect of effective healthcare delivery.
Introduction
Pharmaceutical terminology serves as the backbone of communication within the healthcare sector, encompassing a specialized vocabulary that is crucial for drug development, regulation, and clinical practice. By mastering this terminology, professionals can enhance patient safety, streamline healthcare delivery, and navigate the complexities of the pharmaceutical landscape. Yet, as the industry evolves—driven by technological advancements and emerging health challenges—how can stakeholders keep pace with the ever-changing language of pharmaceuticals? Understanding the key components and historical evolution of this terminology not only sheds light on its significance but also raises critical questions about its future implications in healthcare.
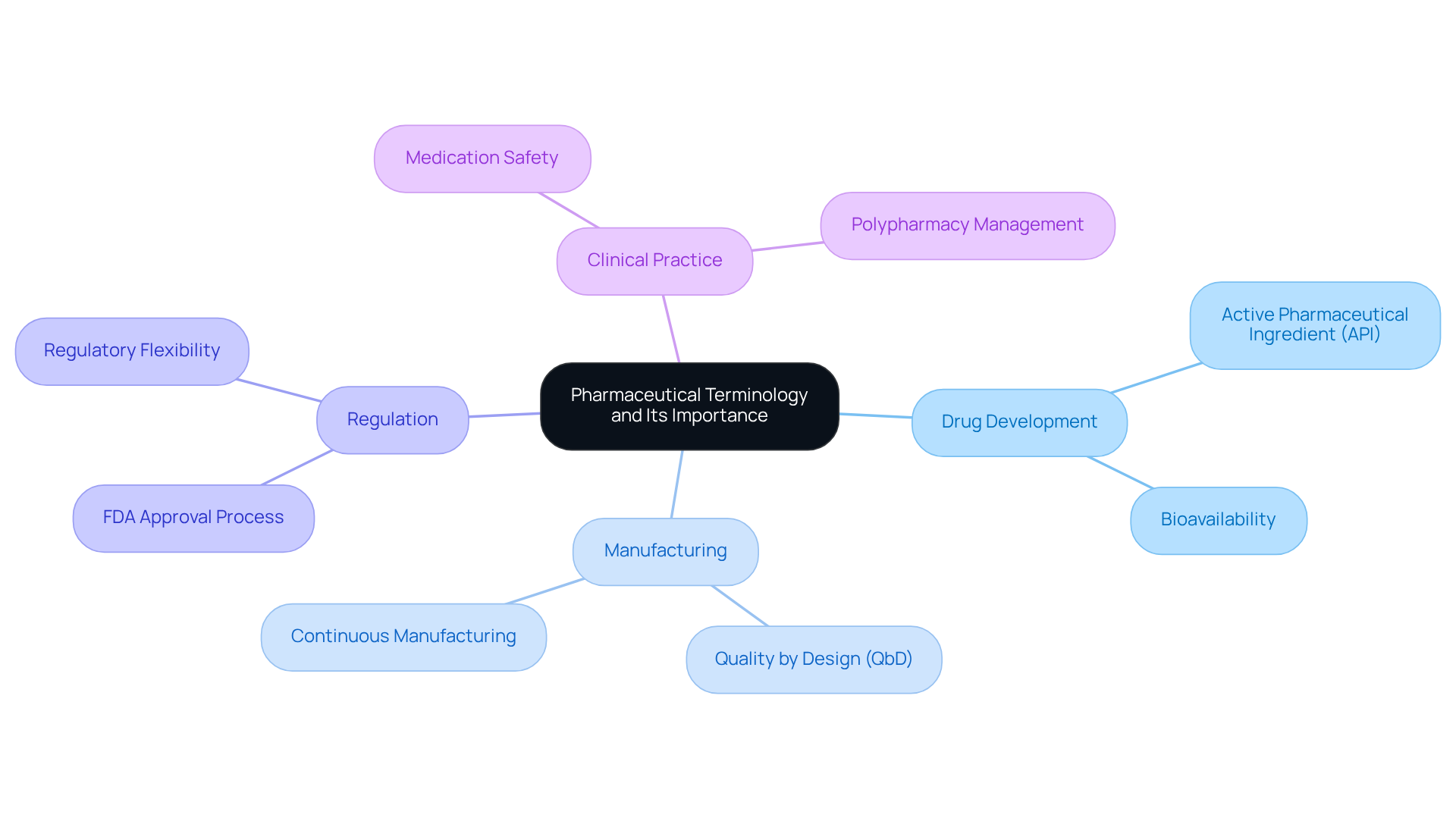
Define Pharmaceutical Terminology and Its Importance
Pharmaceutical terminology represents the specialized vocabulary utilized within , encompassing essential terms related to drug development, manufacturing, regulation, and clinical practice. This language is crucial for facilitating clear communication among healthcare professionals, regulatory bodies, and patients. The use of precise pharmaceutical terminology is vital in preventing misunderstandings that could result in medication errors, enhancing the efficiency of healthcare delivery, and ensuring compliance with regulatory standards.
For instance, concepts such as:
- 'Active Pharmaceutical Ingredient (API)'
- 'bioavailability'
are fundamental when discussing drug formulations and their effects on patients. By prioritizing accurate pharmaceutical terminology, we can significantly improve the safety and effectiveness of healthcare practices.
Trace the Evolution of Pharmaceutical Terminology
The evolution of medical language has its roots in ancient practices, where herbal remedies were documented using rudimentary terms. As pharmacy advanced, particularly during the Renaissance with the establishment of formal pharmacopoeias, the necessity for a consistent pharmaceutical terminology became evident.
The 19th century heralded significant advancements, notably with the introduction of chemical nomenclature, which offered a systematic method for naming compounds. In contemporary times, the evolution of pharmaceutical terminology is constantly shaped by technological innovations, regulatory shifts, and the globalization of .
The emergence of biologics and biosimilars, for instance, has prompted the development of new terms to accurately describe these intricate products. Furthermore, the COVID-19 pandemic has expedited this evolution, underscoring the critical need for precise language in tackling emerging health challenges.
According to PhRMA, member firms have invested over $1 trillion in research and development since 2000, reflecting the sector's commitment to improving drug practices. Additionally, the development of a new drug typically requires an average of 10-15 years and incurs costs around $2.6 billion, with only 12% of new molecular entities receiving FDA approval. This highlights the complexities and challenges inherent in the evolution of medical language.
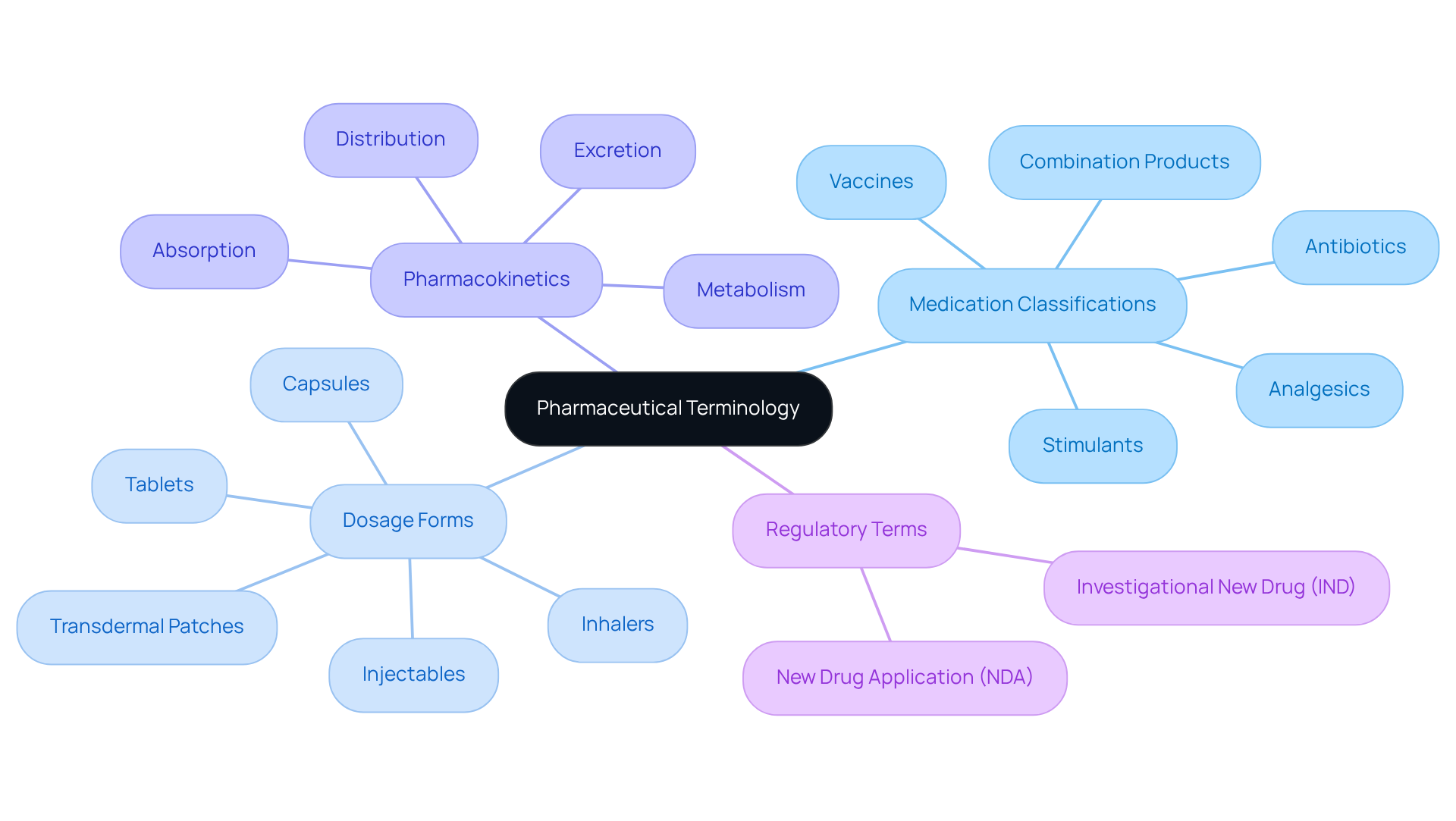
Identify Key Components of Pharmaceutical Terminology
Key elements of pharmaceutical terminology include various categories such as medication classifications, dosage forms, pharmacokinetics, and regulatory terms. Drug classifications are crucial as they categorize medications based on their therapeutic effects, such as analgesics, antibiotics, and stimulants. This categorization facilitates enhanced communication among healthcare professionals and ultimately improves patient care. A significant aspect of medication classifications is , which is updated annually to reflect evolving public health needs and supports formulary development and review for stakeholders. Current trends indicate a rising focus on personalized medicine, which relies significantly on precise medication classifications to tailor treatments to individual patient needs.
Dosage forms refer to the physical presentation of medications, including tablets, capsules, injectables, and emerging forms like transdermal patches and inhalers. These forms are essential for ensuring optimal medication delivery and patient adherence. Pharmacokinetics encompasses terminology that outlines the processes of substance absorption, distribution, metabolism, and excretion (ADME). Understanding these processes is essential for comprehending how compounds interact within the body and their therapeutic effectiveness.
Regulatory definitions, such as 'New Drug Application (NDA)' and 'Investigational New Drug (IND)', are vital for navigating the approval processes for new medications. The significance of these classifications and pharmaceutical terminology cannot be overstated, as they underpin the entire healthcare landscape, directing medication development, ensuring safety, and improving therapeutic outcomes. It is also important to acknowledge ongoing disagreements among experts regarding substance classifications, which adds complexity to this area. By grasping these key components, stakeholders can better navigate the intricacies of the pharmaceutical industry and contribute to enhanced healthcare solutions.
Provide Examples of Pharmaceutical Terminology in Practice
In various facets of the industry, the use of pharmaceutical terminology is essential, particularly during the medication development process. Terms such as 'clinical trial phases'—Phase I, II, and III—are pivotal for delineating the stages involved in assessing a new medication's safety and efficacy. For example, Phase I trials prioritize safety and are conducted with a small group of healthy volunteers, while Phase II trials assess efficacy in a larger patient population. Grasping these phases is vital for stakeholders to adeptly navigate the complexities of drug development, especially given that the overall probability of success (POS) for is estimated at 13.8%.
In the marketing domain, understanding concepts like 'target audience' and 'market segmentation' is imperative for developing impactful promotional strategies for nutraceuticals. Market segmentation statistics indicate that the nutraceutical industry is rapidly evolving, with an increasing focus on personalized health solutions tailored to specific consumer needs.
Furthermore, in regulatory contexts, terms such as 'Good Manufacturing Practices (GMP)' and 'Adverse Drug Reaction (ADR)' are critical for ensuring compliance and safety monitoring. GMP guidelines are instrumental in maintaining product quality throughout the manufacturing process, while ADR reporting is essential for tracking and mitigating potential risks associated with new products. As emphasized by industry experts, comprehending these regulatory terms is crucial for operational success.
These examples highlight the importance of pharmaceutical terminology for fostering effective communication and ensuring operational success within the industry.
Conclusion
Pharmaceutical terminology is foundational to effective communication within the healthcare industry, serving as a critical bridge between various stakeholders, including healthcare professionals, regulatory agencies, and patients. Mastery of this specialized vocabulary not only enhances patient safety but also streamlines healthcare delivery, enabling stakeholders to navigate the complexities of drug development and regulation with confidence.
Throughout the article, key points were explored, including the historical evolution of pharmaceutical terminology from ancient herbal practices to modern classifications shaped by scientific advancements and global market dynamics. The significance of precise terms such as 'Active Pharmaceutical Ingredient' and 'bioavailability' was highlighted, illustrating their role in improving medication safety and efficacy. Additionally, components such as medication classifications, dosage forms, and regulatory definitions were discussed, emphasizing their importance in ensuring compliance and enhancing therapeutic outcomes.
Reflecting on the evolution and components of pharmaceutical terminology underscores its vital role in fostering effective communication and operational success within the industry. As the pharmaceutical landscape continues to evolve with technological advancements and emerging health challenges, stakeholders must remain vigilant in updating their understanding of this terminology. Embracing this knowledge not only supports improved patient care but also contributes to the overall advancement of healthcare practices.
Frequently Asked Questions
What is pharmaceutical terminology?
Pharmaceutical terminology refers to the specialized vocabulary used in the pharmaceutical industry, including terms related to drug development, manufacturing, regulation, and clinical practice.
Why is pharmaceutical terminology important?
It is important for facilitating clear communication among healthcare professionals, regulatory bodies, and patients, preventing misunderstandings that could lead to medication errors, enhancing healthcare delivery efficiency, and ensuring compliance with regulatory standards.
Can you provide examples of pharmaceutical terminology?
Examples include 'Active Pharmaceutical Ingredient (API)' and 'bioavailability,' which are essential when discussing drug formulations and their effects on patients.
How does accurate pharmaceutical terminology impact healthcare practices?
Prioritizing accurate pharmaceutical terminology significantly improves the safety and effectiveness of healthcare practices by minimizing the risk of errors and ensuring clear communication.




