Overview
The article emphasizes the critical importance of sterile packs in nutraceutical manufacturing, highlighting their essential role in preventing contamination and ensuring both product safety and efficacy. It details key characteristics of these packs, including:
- Airtight seals
- Moisture barriers
These features are vital in maintaining product integrity. Furthermore, the discussion includes regulatory requirements and market growth trends that underscore the indispensable function of sterile packs in fostering consumer trust in dietary supplements. This focus on reliability and quality solutions is paramount for industry professionals seeking to enhance their product offerings.
Introduction
The world of nutraceutical manufacturing is increasingly shaped by the critical need for safety and efficacy in dietary supplements. Health-conscious consumers demand products that are both effective and free from contamination. In this context, sterile packs have emerged as essential tools within the industry. These specialized packaging solutions not only safeguard against microbial intrusion but also play a pivotal role in ensuring compliance with stringent health regulations. However, with rapid advancements in packaging technology, how can manufacturers keep pace with evolving standards while maintaining product integrity?
Define Sterile Pack: Essential Characteristics and Purpose
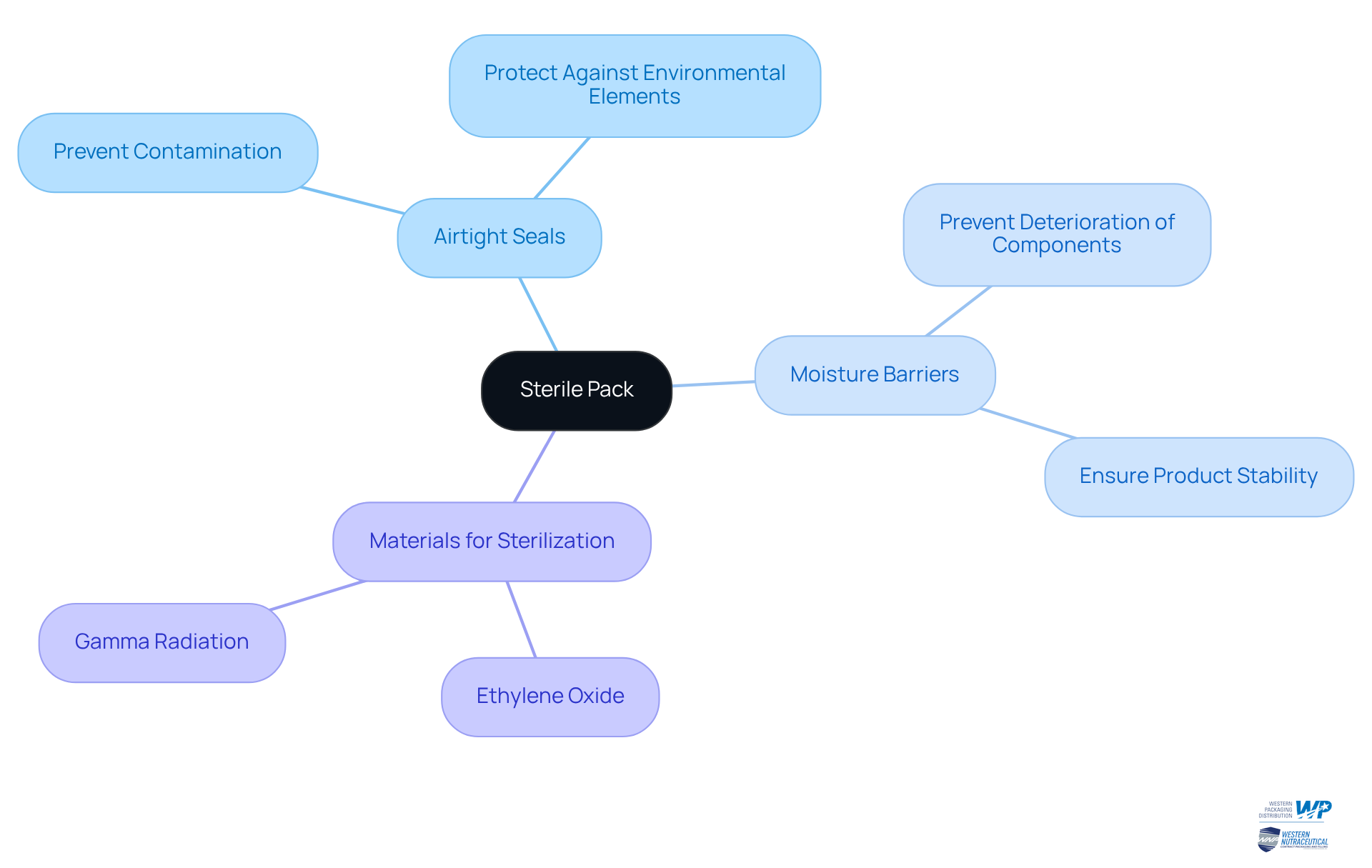
A sterile pack is a specialized packaging solution designed to maintain the cleanliness of its contents, effectively preventing contamination by microorganisms. Key characteristics of a sterile pack include:
- Airtight seals
- Moisture barriers
- Materials that support sterilization processes such as ethylene oxide or gamma radiation
The primary objective of these sterile packs is to ensure that nutraceutical items, including dietary supplements, remain uncontaminated from the point of manufacture to the end-user, thus preserving their efficacy and safety.
Airtight seals play a crucial role, safeguarding against environmental elements that could compromise the integrity of the product. Additionally, moisture barriers are essential in preventing the deterioration of delicate components, ensuring that the items retain their intended benefits throughout their shelf life. Overall, the design and functionality of hygienic containers are vital for protecting health-promoting products and adhering to regulatory standards in the nutraceutical sector.
Contextualize Sterile Packs in Nutraceutical Manufacturing
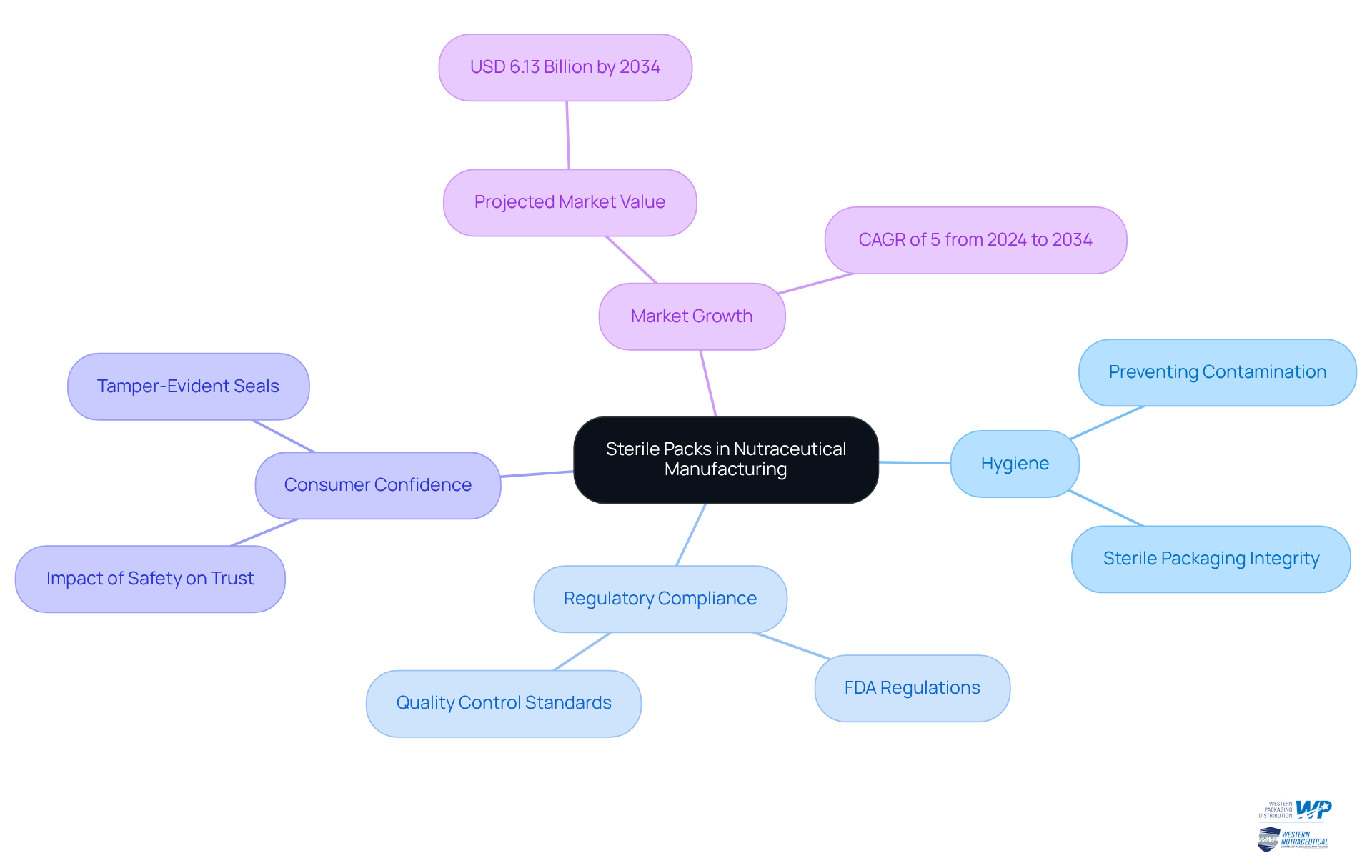
In the nutraceutical production industry, hygienic containers are essential for ensuring safety and adhering to health regulations. As consumer focus on health and wellness intensifies, the demand for safe and effective dietary supplements has surged. Sterile packs serve as an essential barrier against contamination, preventing the intrusion of microorganisms and thus enhancing product integrity.
Regulatory authorities, including the FDA, impose stringent regulations on containers for nutraceuticals, making hygienic wrapping not merely a best practice but a critical requirement for compliance. This necessity underscores the importance of hygienic wrapping in fostering consumer confidence and ensuring the reliability of dietary supplements.
With the nutraceutical container market projected to reach USD 6.13 billion by 2034, expanding at a CAGR of 5% from 2024 to 2034, the role of hygienic wrapping in preserving product quality and consumer trust is paramount.
Trace the Evolution of Sterile Packs: Historical Development and Innovations
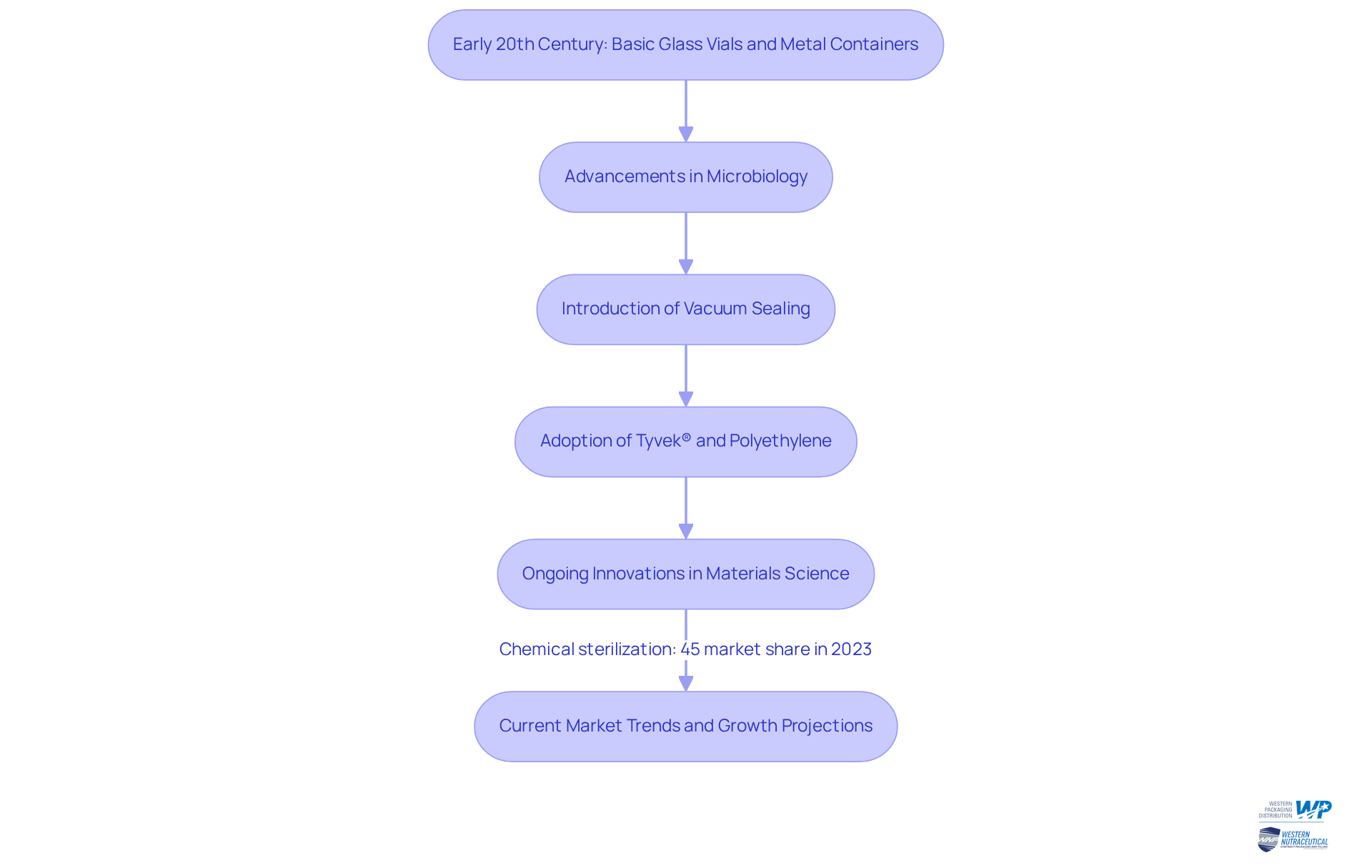
The development of hygienic containers can be traced back to the early 20th century, a period when the significance of sterilization in medical and pharmaceutical applications became widely acknowledged. Initially, basic glass vials and metal containers were utilized; however, as our understanding of microbiology advanced, so too did container technologies. Innovations such as vacuum sealing and the introduction of materials like Tyvek® and polyethylene have significantly enhanced the efficiency of the sterile pack.
Today, ongoing advancements in materials science and sterilization methods continue to foster innovation, resulting in more efficient and dependable solutions that meet the specific requirements of the nutraceutical industry. The rising demand for hygienic containment options, such as a sterile pack, is primarily driven by increased awareness of infection management, which is essential for ensuring product safety and compliance with regulatory standards.
Furthermore, the sterile medical containers market is projected to experience substantial growth, with chemical sterilization accounting for over 45% of the market share in 2023. Key players such as DuPont and Amcor are leading these innovations, contributing to the development of advanced packaging technologies that enhance the safety and efficacy of dietary supplements.
Examine Components and Variations of Sterile Packs: Types and Applications
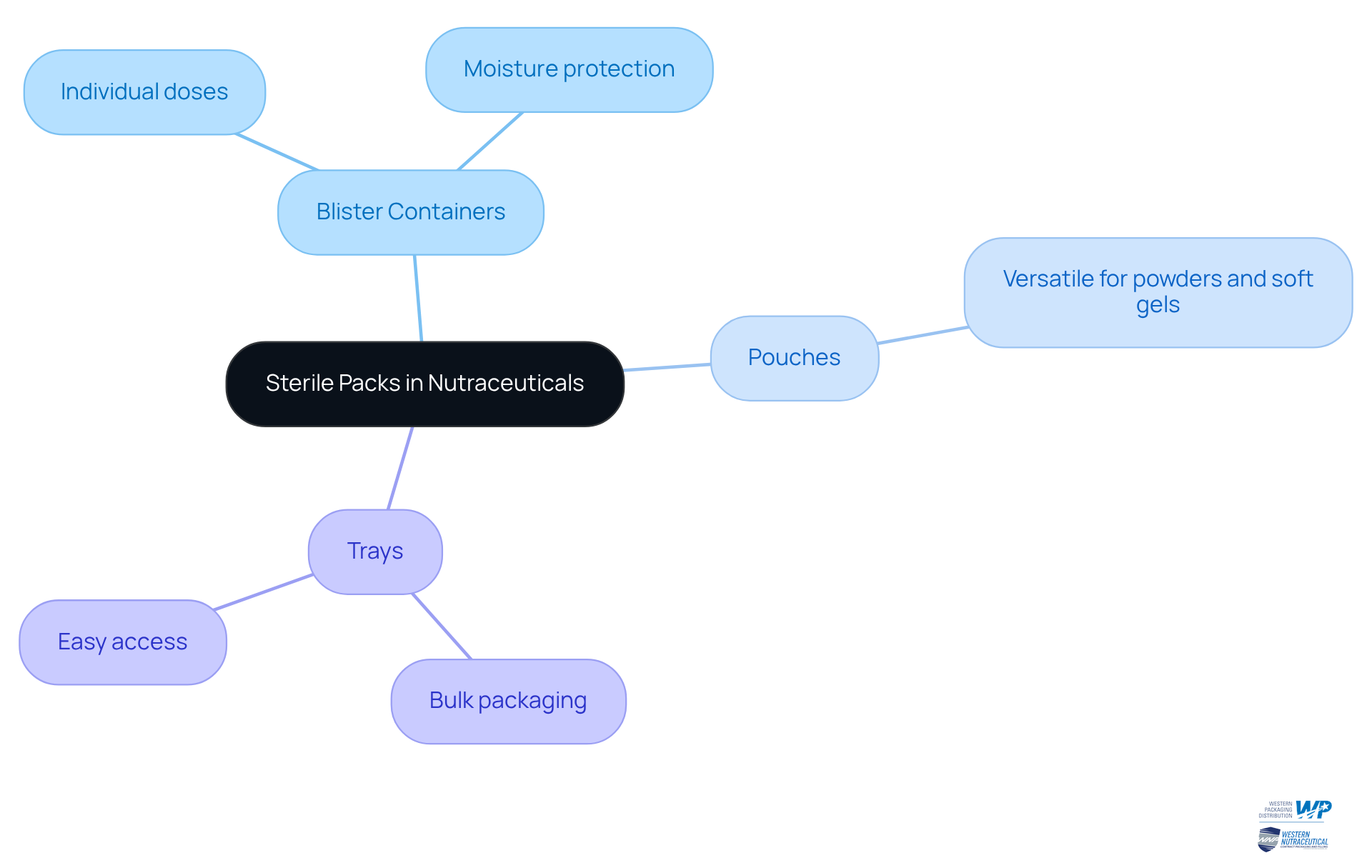
Sterile bundles are available in various forms, each meticulously crafted for specific applications within the nutraceutical industry. Prominent varieties include:
- Blister containers
- Pouches
- Trays
Blister packs are frequently utilized for individual doses of supplements, offering exceptional protection against moisture and light. In contrast, pouches are versatile, accommodating a range of items from powders to soft gels. Trays typically serve bulk packaging needs and are designed to facilitate easy access to individual units. Each type of sterile pack is designed to meet the unique requirements of various nutraceutical products, ensuring their safety and efficacy during their shelf life.
Conclusion
A thorough understanding of sterile packs reveals their critical role in the nutraceutical manufacturing process. These specialized packaging solutions are designed to maintain the integrity and safety of dietary supplements, ensuring they remain free from contamination and effective for consumer use. The essential characteristics of sterile packs, such as airtight seals and moisture barriers, are not merely technical specifications; they are fundamental to preserving the quality and efficacy of health-promoting products.
Throughout the article, key insights have been highlighted, including the historical evolution of sterile packs, their various types and applications, and the regulatory framework that mandates their use in the nutraceutical industry. Innovations in materials and sterilization methods have significantly advanced the capabilities of these packaging solutions, making them indispensable in today’s health-conscious market. As the demand for safe and effective dietary supplements continues to grow, so does the importance of adhering to strict packaging standards.
In light of the critical function that sterile packs serve, it is essential for manufacturers to prioritize their implementation in the production process. By investing in advanced sterile packaging technologies, companies not only comply with regulatory requirements but also enhance consumer trust and product reliability. As the nutraceutical market expands, embracing the significance of sterile packs will be vital for ensuring the safety and efficacy of dietary supplements, ultimately contributing to a healthier society.
Frequently Asked Questions
What is a sterile pack?
A sterile pack is a specialized packaging solution designed to maintain the cleanliness of its contents and prevent contamination by microorganisms.
What are the essential characteristics of a sterile pack?
Key characteristics of a sterile pack include airtight seals, moisture barriers, and materials that support sterilization processes such as ethylene oxide or gamma radiation.
What is the primary purpose of sterile packs?
The primary purpose of sterile packs is to ensure that nutraceutical items, including dietary supplements, remain uncontaminated from the point of manufacture to the end-user, preserving their efficacy and safety.
How do airtight seals contribute to the effectiveness of sterile packs?
Airtight seals safeguard against environmental elements that could compromise the integrity of the product.
Why are moisture barriers important in sterile packs?
Moisture barriers are essential for preventing the deterioration of delicate components, ensuring that the items retain their intended benefits throughout their shelf life.
How do sterile packs relate to regulatory standards in the nutraceutical sector?
The design and functionality of hygienic containers, such as sterile packs, are vital for protecting health-promoting products and adhering to regulatory standards in the nutraceutical sector.




