Overview
This article delineates five essential steps for effective supplement contract manufacturing. It underscores the significance of:
- Initial consultation
- Formulation development
- Ingredient sourcing
- Manufacturing planning
- Quality control
Each of these steps is vital in ensuring that dietary supplements are produced efficiently, comply with regulatory standards, and meet consumer demands. By adhering to these practices, manufacturers can enhance the overall quality and market readiness of their products.
Introduction
The nutraceutical industry is experiencing remarkable growth, with the global market for dietary supplements projected to surpass USD 159 billion in the near future. This surge in demand underscores the essential role of supplement contract manufacturing, a strategic approach that enables brands to concentrate on innovation while capitalizing on the expertise of specialized producers.
However, navigating the complexities of this manufacturing process prompts critical inquiries:
- How can companies ensure they select the right partner?
- What steps are vital for maintaining quality and compliance?
This article explores the five key steps for effective supplement contract manufacturing, offering insights that can empower brands to excel in a competitive landscape.
Define Contract Manufacturing in the Nutraceutical Industry
Supplement contract manufacturing in the nutraceutical sector involves outsourcing the creation of dietary supplements to specialized external producers. This strategic approach allows brands to leverage the expertise, advanced facilities, and cutting-edge technology of established manufacturers, ensuring high-quality output while focusing on their core business functions. Western Packaging provides that optimize your supply chain through customized solutions, including:
- Warehousing
- Inventory management
- Logistics
Contract manufacturers manage the entire process, from formulation and ingredient sourcing to manufacturing and packaging, facilitating a streamlined path to market.
The global nutraceutical manufacturing services market is projected to reach USD 159.26 billion in 2023, with a compound annual growth rate (CAGR) of 12.04% anticipated through 2030. This growth reflects the rising demand for dietary supplements, fueled by increasing health consciousness among consumers and the need for effective solutions to dietary deficiencies.
Outsourcing manufacturing presents several key advantages, including:
- Cost-effectiveness
- Scalability
- Access to specialized knowledge
By partnering with production experts, brands can reduce overhead costs and mitigate risks associated with manufacturing, such as compliance with stringent regulations. Industry leaders emphasize that harnessing the capabilities of manufacturing partners not only enhances operational efficiency but also accelerates time-to-market for new products.
In conclusion, supplement contract manufacturing is a crucial element in the nutraceutical landscape, enabling brands to deliver high-quality dietary supplements while concentrating on innovation and market expansion. With integrated filling services for various products, such as powders, gummies, and soft gels, Western Packaging enhances brand appeal and recognition through innovative packaging design solutions. This seamless integration of design, filling, and logistics ensures that nutraceutical producers can optimize their supply chain management while enhancing their product's shelf appeal.
Outline the Steps in the Contract Manufacturing Process
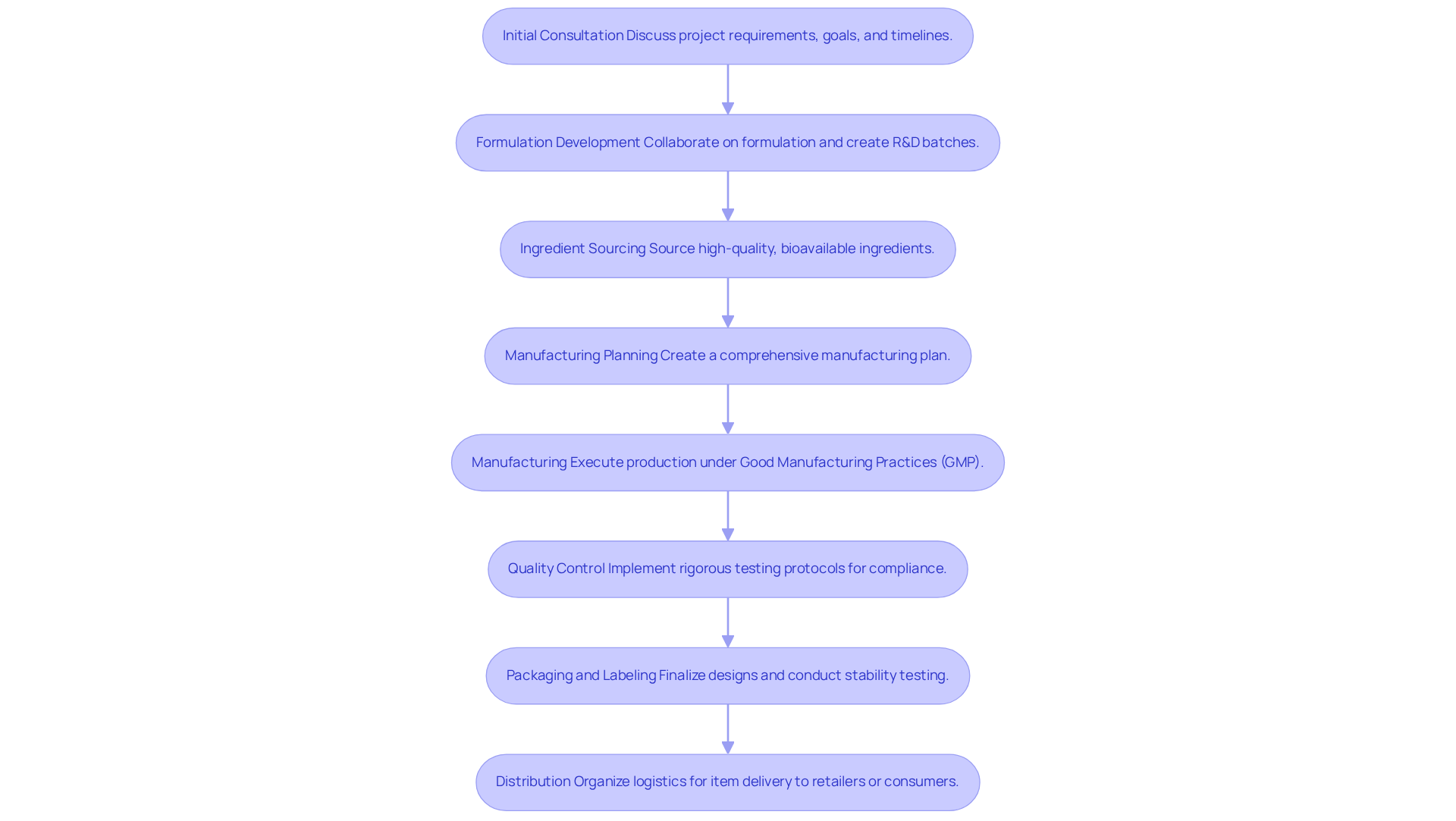
The contract manufacturing process for nutraceuticals unfolds through several key steps, each designed to ensure efficiency and compliance:
- Initial Consultation: This stage involves a thorough discussion of project requirements, goals, and timelines with the manufacturer. Establishing clear expectations early on is crucial for a successful partnership.
- Formulation Development: Collaboration on formulation is essential, ensuring alignment with market needs and adherence to regulatory standards. This stage frequently involves generating small R&D batches to verify formulation viability before large-scale manufacturing.
- Ingredient Sourcing: Manufacturers leverage established relationships with trusted suppliers to source high-quality, bioavailable ingredients. This step is vital for ensuring that the final product meets both efficacy and safety standards.
- Manufacturing Planning: A comprehensive manufacturing plan is created, detailing timelines, batch sizes, and resource distribution. This planning phase enhances operational efficiency and accommodates seasonal demands.
- Manufacturing: The creation process is executed in accordance with (GMP), ensuring that all operations meet stringent quality and safety benchmarks. Quality checks during this phase include content uniformity and microbiological testing, highlighting Western Packaging's commitment to excellence.
- Quality Control: Rigorous testing protocols are implemented to verify item quality and compliance with regulatory requirements. Only materials that pass identity, potency, and safety tests are released into inventory for production, reflecting Western Packaging's high standards.
- Packaging and Labeling: Finalizing packaging designs and ensuring that labels meet regulatory requirements is crucial for market readiness. This step also involves stability testing to determine item shelf life and ensure potency, utilizing innovative packaging design solutions by Western Packaging to enhance brand recognition and shelf appeal.
- Distribution: The final step organizes logistics for item delivery, whether to retailers or directly to consumers. Effective distribution strategies minimize lead times and enhance inventory replenishment, optimizing the supply chain with Western Packaging's comprehensive 3PL services.
By following these steps, businesses can streamline their contract manufacturing processes, ensuring timely delivery of high-quality dietary supplements that meet consumer demands and regulatory standards.
Ensure Compliance with Regulatory Standards and Quality Control
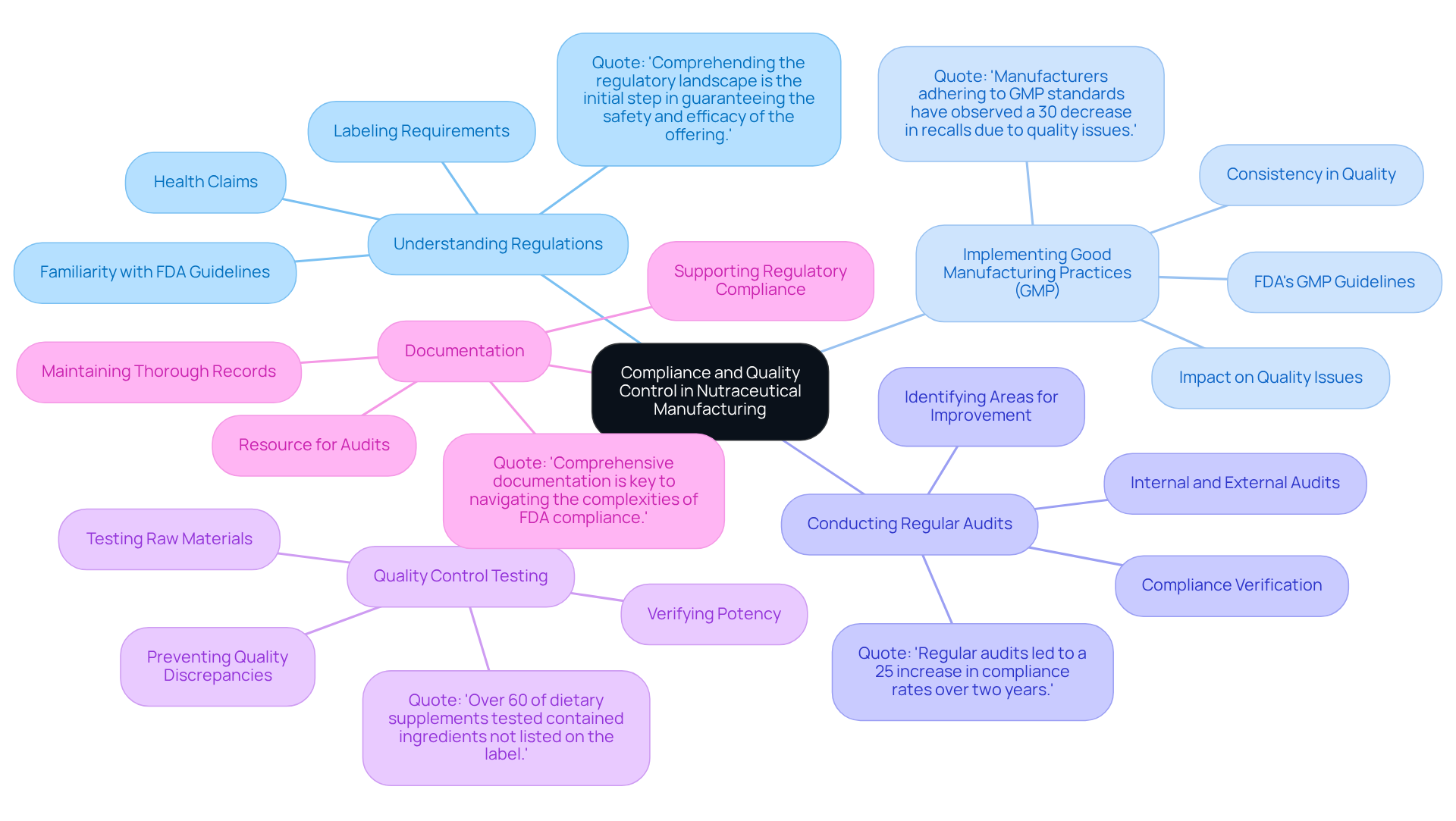
Ensuring compliance with regulatory standards is paramount for the success of nutraceutical manufacturing. To achieve this, several key practices must be implemented:
- Understanding Regulations: Familiarizing oneself with FDA regulations and guidelines specific to dietary supplements, including labeling requirements and permissible health claims, is essential. This foundational knowledge aids in compliance and helps avoid potential pitfalls. As industry specialists emphasize, "Comprehending the regulatory landscape is the initial step in guaranteeing the safety and efficacy of the offering."
- Implementing Good Manufacturing Practices (GMP): Adhering to GMP is vital for ensuring consistent quality and safety in production. The FDA's GMP guidelines delineate the necessary conditions and practices for creating dietary supplements, significantly influencing quality. Companies that rigorously follow GMP protocols often report fewer quality issues and enhanced consumer trust. A recent study found that "Manufacturers adhering to GMP standards have observed a 30% decrease in recalls due to quality issues."
- Conducting Regular Audits: Performing both internal and external audits is crucial for verifying compliance with industry standards. These audits identify areas for improvement and ensure that all processes align with regulatory requirements, thereby minimizing risks associated with non-compliance. A case study from a prominent nutraceutical producer revealed that regular audits led to a 25% increase in compliance rates over two years.
- Quality Control Testing: Implementing rigorous testing protocols at various stages of production is essential for ensuring ingredient purity and safety. For instance, testing raw materials for contaminants and verifying the potency of active ingredients can prevent quality discrepancies that may lead to adverse consumer reactions. Statistics indicate that "over 60% of dietary supplements tested contained ingredients not listed on the label, highlighting the need for stringent quality control measures."
- Documentation: Maintaining thorough records of all processes, tests, and compliance measures is critical for transparency and accountability. Proper documentation not only supports regulatory compliance but also serves as a valuable resource during audits and inspections, demonstrating a commitment to quality and safety. Regulatory experts stress that "Comprehensive documentation is key to navigating the complexities of FDA compliance."
By integrating these practices, nutraceutical producers can significantly enhance their adherence to FDA regulations, ultimately resulting in superior quality and increased consumer trust.
Select the Right Contract Manufacturer: Key Evaluation Criteria
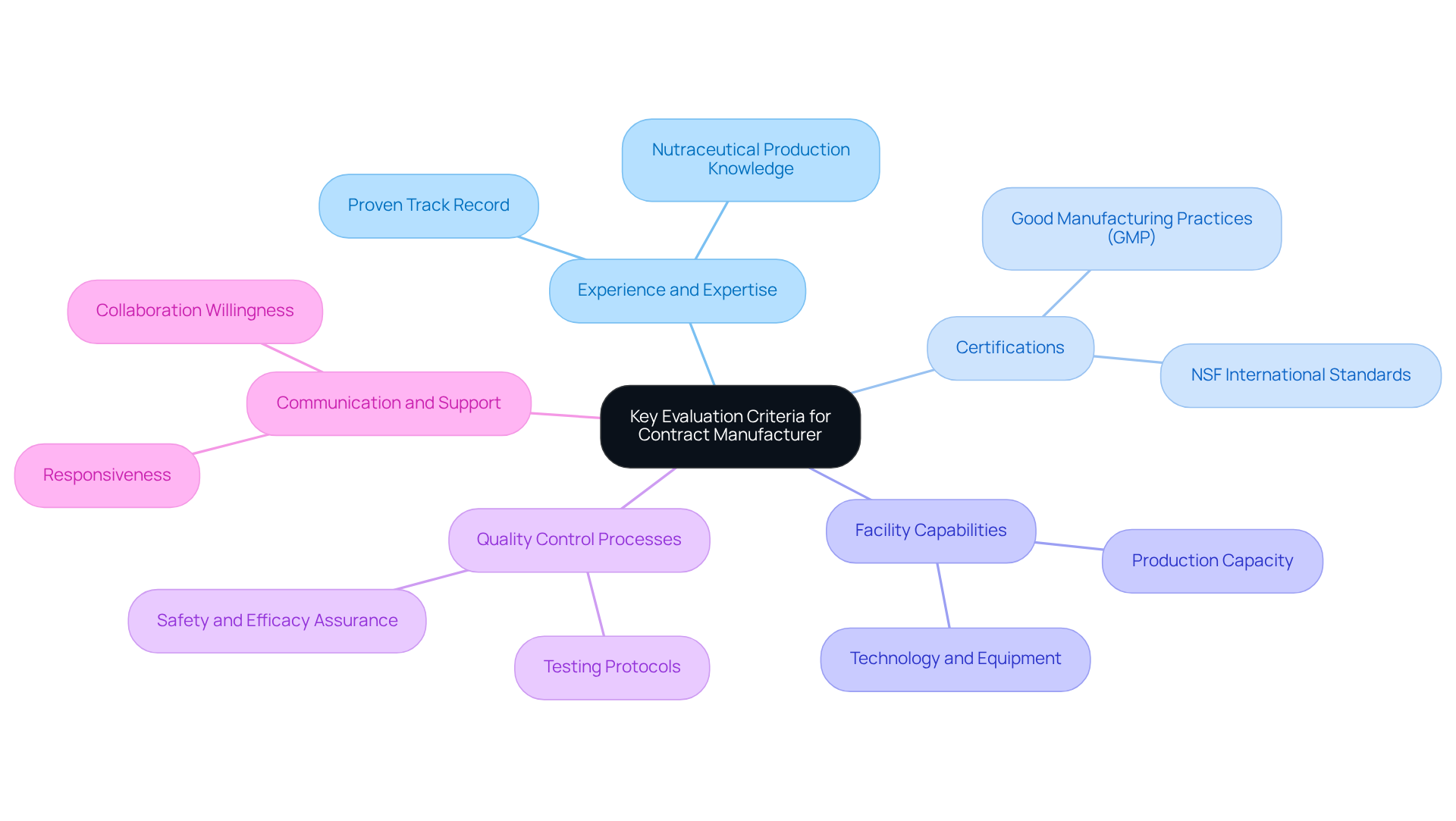
When selecting a contract manufacturer for dietary supplements, it is essential to consider several key evaluation criteria to ensure a successful partnership:
- Experience and Expertise: Prioritize manufacturers with a proven track record in producing nutraceuticals that align with your product specifications. The typical experience level of contract producers in this sector is significant, as it reflects their ability to navigate the complexities of formulation and creation.
- Certifications: Confirm that the producer possesses relevant certifications, such as Good Manufacturing Practices (GMP) and NSF International standards. These certifications are essential signs of a producer’s dedication to upholding high-quality manufacturing standards. As Debashree Bora observes, "Stringent quality standards and certifications such as cGMP and NSF further highlight the significance of specialized production companies."
- Facility Capabilities: Assess the producer’s production capabilities, including the technology and equipment they utilize. Understanding their capacity to meet your volume requirements is crucial, especially as the nutraceutical supplement contract manufacturing services market is projected to grow significantly, reaching USD 386.6 billion by 2033, with a CAGR of 12.0% from 2024 to 2029.
- Quality Control Processes: Inquire about the producer’s quality control measures and testing protocols. Robust quality assurance processes are vital for ensuring product safety and efficacy, particularly in a market where consumer demand for high-quality, innovative dietary supplements and supplement contract manufacturing is increasing. The increasing inclination towards vegan, organic, and minimally processed nutraceuticals is transforming the market, urging producers to adjust their production methods accordingly.
- Communication and Support: Evaluate the supplier’s responsiveness and willingness to collaborate throughout the production process. Effective communication is vital for addressing any concerns and ensuring that your development aligns with market needs.
By concentrating on these standards, companies can make informed choices when choosing a production partner, ultimately improving their offerings and market presence. Furthermore, it is essential to recognize challenges like intellectual property threats and regulatory intricacies that may occur in the manufacturing environment, along with the increasing influence of e-commerce on distribution pathways for nutraceutical items.
Foster Communication and Collaboration with Your Manufacturer
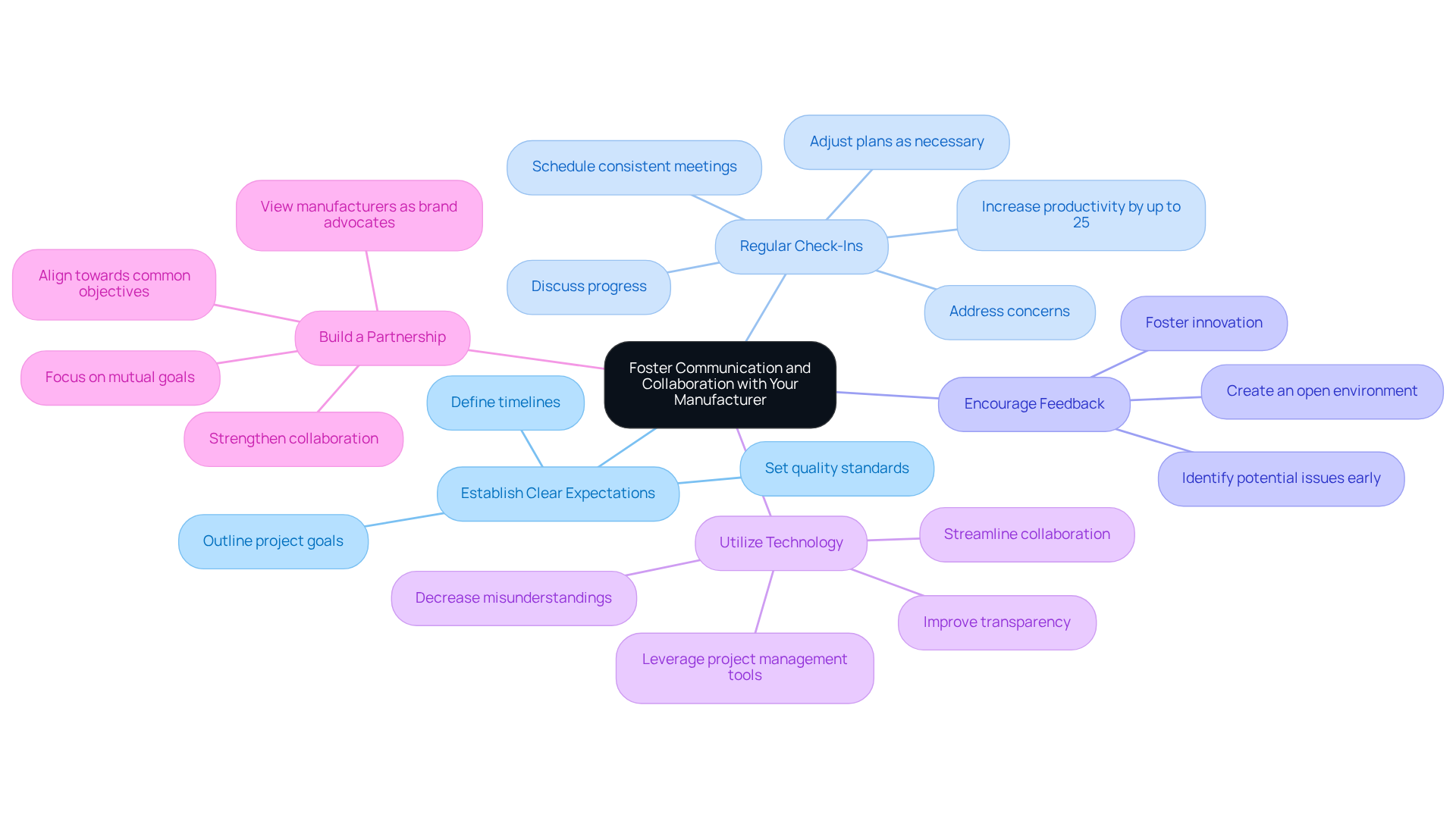
To foster effective communication and collaboration with your contract manufacturer, consider the following strategies:
- Establish Clear Expectations: Clearly outline project goals, timelines, and quality standards from the outset. This ensures that both parties are aligned and understand the objectives.
- Regular Check-Ins: Schedule consistent meetings to discuss progress, address concerns, and make necessary adjustments. Frequent check-ins can greatly improve success rates in manufacturing agreements by ensuring everyone is aligned. In fact, teams that communicate effectively see an employee productivity increase of up to 25%.
- Encourage Feedback: Create an open environment where both parties can provide feedback and suggestions for improvement. This collaborative approach fosters innovation and helps identify potential issues early.
- Utilize Technology: Leverage project management tools and communication platforms to streamline collaboration and keep everyone informed. Effective use of technology can improve transparency and decrease misunderstandings, which are common pain points in manufacturing agreements.
- Build a Partnership: Approach the relationship as a partnership, focusing on mutual goals and shared success. This mindset not only strengthens the collaboration but also aligns both parties towards achieving common objectives. As one industry leader observed, "The top production partners play a role beyond a supply chain vendor." They are brand supporters and regard your items as though they were their own.
By applying these strategies, producers can enhance their cooperation with external manufacturers, resulting in improved product quality and operational efficiency. Additionally, it's important to recognize that 86% of employees cite ineffective communication and poor internal communication skills for workplace failures, underscoring the need for effective communication in contract manufacturing.
Conclusion
Supplement contract manufacturing plays a pivotal role in the nutraceutical industry, providing brands with the opportunity to deliver high-quality dietary supplements while focusing on their core business objectives. By outsourcing production to specialized manufacturers, companies can tap into advanced technologies and expertise, ultimately enhancing product quality and operational efficiency.
The article outlines essential steps in the contract manufacturing process, emphasizing the importance of:
- Initial consultations
- Formulation development
- Stringent quality control measures
Key factors such as:
- Selecting the right manufacturer
- Understanding regulatory compliance
- Fostering effective communication
are critical to ensuring a successful partnership. By adhering to these practices, brands can not only meet consumer demand but also navigate the complexities of the nutraceutical market.
In a landscape where health consciousness is on the rise, the significance of effective supplement contract manufacturing cannot be overstated. Brands are encouraged to leverage the advantages of outsourcing, ensuring they remain competitive while delivering innovative and compliant products. By prioritizing collaboration and quality, businesses can build strong partnerships that drive growth and enhance their market presence in the ever-evolving nutraceutical sector.
Frequently Asked Questions
What is contract manufacturing in the nutraceutical industry?
Contract manufacturing in the nutraceutical industry involves outsourcing the creation of dietary supplements to specialized external producers, allowing brands to leverage the expertise and advanced facilities of established manufacturers.
What are the advantages of outsourcing manufacturing in the nutraceutical sector?
Key advantages include cost-effectiveness, scalability, and access to specialized knowledge, which help brands reduce overhead costs and comply with regulations.
What is the projected growth of the nutraceutical manufacturing services market?
The global nutraceutical manufacturing services market is projected to reach USD 159.26 billion in 2023, with a compound annual growth rate (CAGR) of 12.04% anticipated through 2030.
What are the steps involved in the contract manufacturing process for nutraceuticals?
The steps include: 1. Initial Consultation 2. Formulation Development 3. Ingredient Sourcing 4. Manufacturing Planning 5. Manufacturing 6. Quality Control 7. Packaging and Labeling 8. Distribution
What happens during the initial consultation in the contract manufacturing process?
The initial consultation involves a thorough discussion of project requirements, goals, and timelines with the manufacturer to establish clear expectations.
How is formulation developed in the contract manufacturing process?
Formulation development involves collaboration to ensure the product aligns with market needs and regulatory standards, often including small R&D batches to verify viability.
Why is ingredient sourcing important in contract manufacturing?
Ingredient sourcing is crucial for ensuring high-quality, bioavailable ingredients are used, which is essential for the efficacy and safety of the final product.
What practices are followed during the manufacturing phase?
The manufacturing phase adheres to Good Manufacturing Practices (GMP), ensuring operations meet stringent quality and safety benchmarks, including quality checks for content uniformity and microbiological testing.
What does quality control involve in the contract manufacturing process?
Quality control involves rigorous testing protocols to verify product quality and compliance with regulatory requirements, ensuring only materials that pass tests are used in production.
How is packaging and labeling handled in the contract manufacturing process?
Packaging and labeling finalization ensures designs meet regulatory requirements and involves stability testing to determine shelf life and potency.
What is the final step in the contract manufacturing process?
The final step is distribution, which organizes logistics for delivering products to retailers or directly to consumers, optimizing the supply chain.




