Overview
Serialization in pharmaceutical packaging is a critical process that involves assigning a unique serial number to each saleable unit of medication. This practice is essential for tracking products throughout the supply chain and plays a vital role in preventing counterfeit drugs. Not only does this process ensure regulatory compliance and enhance product safety, but it also significantly aids in inventory management. The implications for public health and operational efficiency in the pharmaceutical industry are profound, underscoring the necessity of implementing robust serialization practices.
Introduction
Serialization in pharmaceutical packaging represents a critical process that assigns unique identifiers to drug units, thereby ensuring their traceability throughout the supply chain. This essential practice not only enhances product safety and inventory management but also plays a pivotal role in combating the pervasive issue of counterfeit medications, which pose significant risks to public health. As regulatory pressures mount and compliance deadlines approach, pharmaceutical companies face the daunting challenge of implementing effective serialization strategies.
How can these organizations navigate the complexities of serialization while safeguarding both their operations and consumer trust?
Define Serialization in Pharmaceutical Packaging
What is serialization pharmaceutical packaging? It is the process of assigning a unique serial number to each saleable unit of a drug. This distinctive identifier, typically printed on the packaging, is essential for tracking and tracing items throughout the supply chain. Not only does serialization enhance item identification, but understanding what is serialization pharmaceutical packaging also plays a vital role in ensuring compliance with regulatory requirements designed to prevent counterfeit medications from entering the market. Each serialized unit contains information about the product's origin, batch number, and expiration date, facilitating improved inventory management and product safety.
Around 80% of nations have mandated tracking systems to mitigate the dangers associated with counterfeit medicines, which may account for up to 30% of pharmaceuticals sold in developing countries, according to the World Health Organization. Notably, Australia is among the remaining 20% of nations that have yet to fully implement tracking regulations. The execution of data packaging has been crucial in regions such as the EU and the U.S., where what is serialization pharmaceutical packaging is required by the Drug Supply Chain Security Act (DSCSA), mandating that all prescription drug packages display a unique identifier by November 27, 2023. This compliance not only safeguards consumers but also optimizes supply chain operations, enabling manufacturers to efficiently manage their inventory and reduce the risk of drug diversion and theft.
Practical examples of effective implementation include collaborations between companies like Arvato Systems and Advanco, which enhance compliance and operational efficiency in the healthcare sector by providing tailored solutions that meet regulatory requirements. By adopting a systematic approach, pharmaceutical firms can significantly bolster their supply chain integrity and protect public health. However, it is crucial to acknowledge that implementing data formatting can be complex and costly, with each individual production line necessitating new equipment that can exceed $500,000 AUD. The primary drawback of data formatting is the , which producers must consider when formulating their compliance strategies.
Explain the Importance of Serialization in Preventing Counterfeiting
The significance of what is serialization pharmaceutical packaging in combating counterfeiting cannot be overstated. Counterfeit drugs represent a serious threat to public health, resulting in ineffective treatments and potential harm to patients.
What is serialization pharmaceutical packaging offers a robust method for of medicinal products. By assigning a unique identifier to each unit, stakeholders throughout the distribution chain can effectively track items from manufacturing to the point of sale, illustrating what is serialization pharmaceutical packaging.
This traceability ensures that only authorized products reach consumers, thereby enhancing safety and trust within the healthcare supply chain. Moreover, the systematic organization of information assists businesses in complying with stringent regulations, significantly reducing the risk of legal repercussions associated with counterfeit goods.
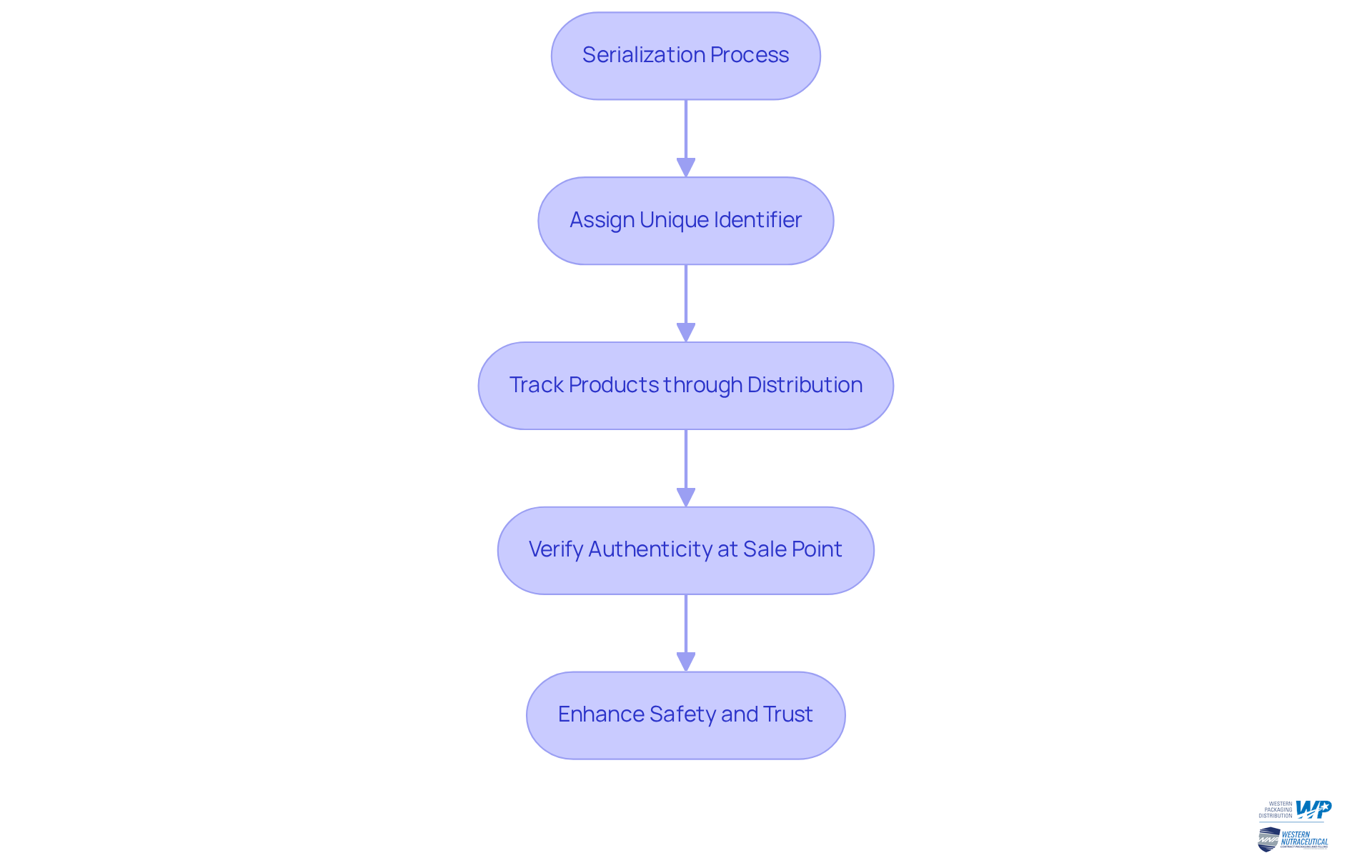
Outline Regulatory Requirements for Serialization in Pharma
Regulatory standards for tracking in the drug industry are significantly shaped by the Drug Supply Chain Security Act (DSCSA) in the United States and the Falsified Medicines Directive (FMD) in the European Union. These regulations require pharmaceutical manufacturers to understand what is serialization pharmaceutical packaging, which involves assigning a unique serial number to each saleable unit of medication that must be meticulously recorded and maintained throughout the supply chain.
Compliance entails not only the proper numbering of items but also understanding what is serialization pharmaceutical packaging, which involves implementing robust systems for monitoring and tracing these items. Noncompliance can lead to severe repercussions, including fines reaching up to $500,000 per violation, product seizures, and restrictions on market access.
For instance, a mid-sized drug company achieved full DSCSA compliance in under 90 days, contrasting with the typical 4-6 month implementation timeline for most producers. This underscores the critical importance of for companies aiming to operate lawfully and ethically.
Furthermore, organizations must evaluate their compliance readiness as the final enforcement deadlines draw near—November 27, 2025, for large dispensers and November 27, 2026, for smaller dispensers. Implementing effective data structuring strategies is essential to ensure seamless product flow and safeguard patient safety. Additionally, real-time verification tools, such as verification router services (VRS) and authenticated trade partner tokens (ATP), play a vital role in maintaining compliance and protecting the integrity of the drug distribution network.
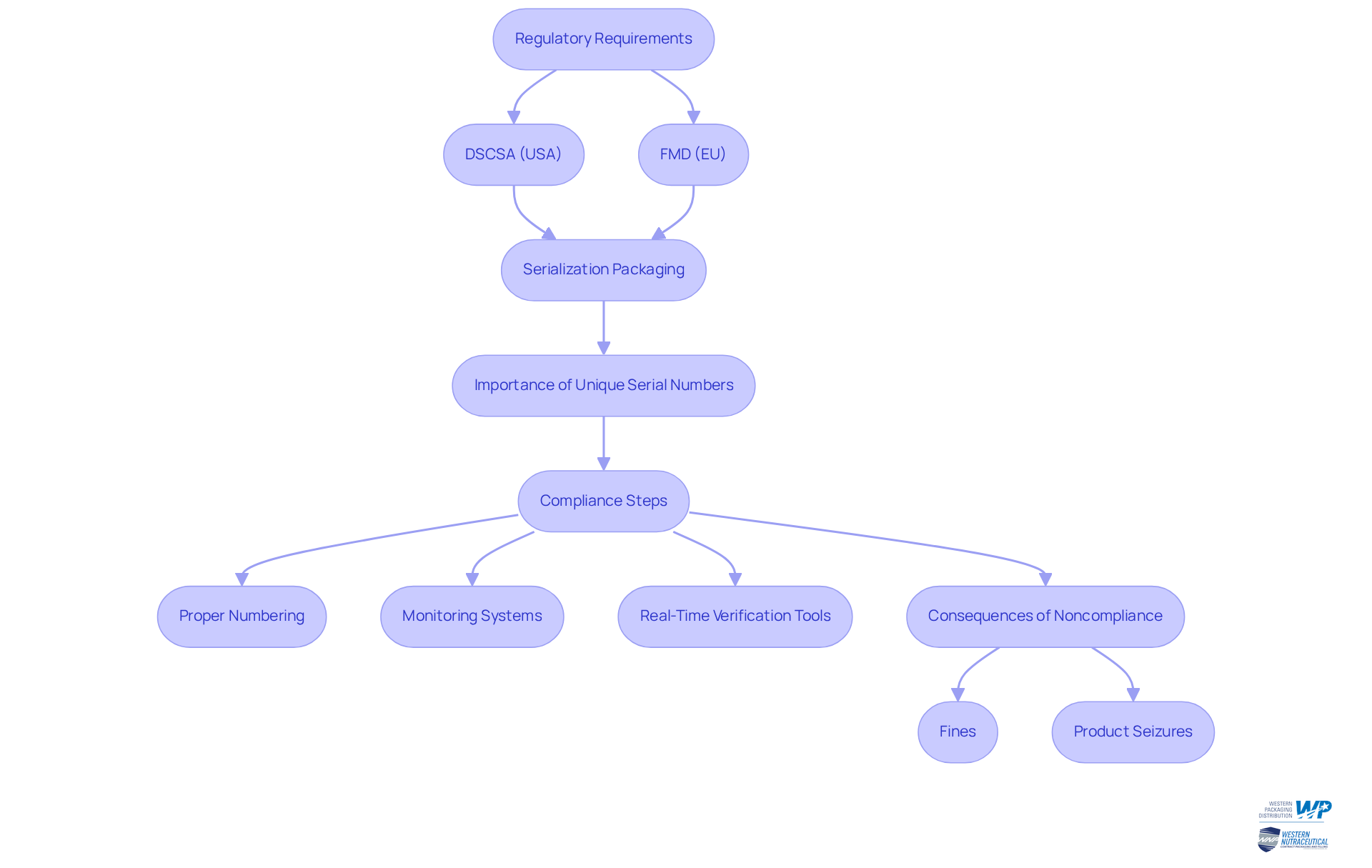
Describe Methods and Technologies for Implementing Serialization
Implementing what is serialization pharmaceutical packaging is essential for ensuring accuracy and efficiency. Various methods and technologies are designed to achieve this goal, with common approaches including the use of (Radio-Frequency Identification) tags.
- Barcodes and QR codes stand out for their cost-effectiveness and ease of integration into existing packaging lines. These codes can be scanned at different locations in the supply chain, confirming authenticity and monitoring movement.
- Conversely, RFID technology, while more expensive, offers enhanced capabilities for real-time tracking and inventory management.
- Moreover, software solutions play a crucial role in managing the data linked to serialized items, ensuring that all information is precisely documented and readily available.
- To maximize the effectiveness of serialization processes, companies must also invest in staff training and system testing. This investment is vital to execute serialization flawlessly, thereby minimizing the risk of errors that could compromise product integrity.
Conclusion
Serialization in pharmaceutical packaging serves as a crucial safeguard against the pervasive issue of counterfeit drugs, ensuring that each medication unit is uniquely identifiable throughout its journey from production to consumer. This system not only enhances product traceability but also aligns with regulatory mandates aimed at protecting public health. By understanding and implementing serialization, pharmaceutical companies can significantly improve their operational efficiency while ensuring compliance with stringent laws designed to uphold the integrity of the drug supply chain.
Key insights from the discussion underscore the importance of serialization as a means to combat counterfeiting, highlighting the role of unique identifiers in verifying product authenticity. The article has explored various methods and technologies, such as:
- Barcodes
- QR codes
- RFID systems
which facilitate effective serialization. Additionally, the regulatory landscape—including the Drug Supply Chain Security Act and the Falsified Medicines Directive—outlines the necessity for compliance to avoid severe penalties and enhance patient safety.
Ultimately, the implementation of serialization in pharmaceutical packaging is not merely a regulatory requirement but a vital practice that fosters trust and safety in healthcare. As the industry moves towards stricter compliance deadlines, stakeholders are encouraged to adopt robust serialization strategies and invest in the necessary technologies and training. This proactive approach will not only protect consumers but also fortify the entire pharmaceutical supply chain against the threats posed by counterfeit medications.
Frequently Asked Questions
What is serialization in pharmaceutical packaging?
Serialization in pharmaceutical packaging is the process of assigning a unique serial number to each saleable unit of a drug, which is printed on the packaging for tracking and tracing throughout the supply chain.
Why is serialization important in the pharmaceutical industry?
Serialization is essential for enhancing item identification, ensuring compliance with regulatory requirements to prevent counterfeit medications, and facilitating improved inventory management and product safety.
How widespread is the implementation of serialization tracking systems globally?
Approximately 80% of nations have mandated tracking systems to combat counterfeit medicines, while around 20% of nations, including Australia, have yet to fully implement such regulations.
What regulatory act requires serialization in the U.S.?
The Drug Supply Chain Security Act (DSCSA) requires that all prescription drug packages in the U.S. display a unique identifier by November 27, 2023.
What are the benefits of compliance with serialization regulations?
Compliance with serialization regulations safeguards consumers, optimizes supply chain operations, enables efficient inventory management, and reduces the risk of drug diversion and theft.
Can you provide examples of effective serialization implementation?
Collaborations between companies like Arvato Systems and Advanco have enhanced compliance and operational efficiency in the healthcare sector by providing tailored solutions that meet regulatory requirements.
What challenges do pharmaceutical companies face when implementing serialization?
Implementing serialization can be complex and costly, with individual production lines requiring new equipment that can exceed $500,000 AUD, which poses a significant financial challenge for producers.
What is the main drawback of data formatting in serialization?
The primary drawback of data formatting in serialization is the substantial upfront and ongoing costs that producers must consider when developing their compliance strategies.




