Overview
The article delineates a five-step process for implementing a track and trace system in pharmaceutical packaging, underscoring its critical role in ensuring supply chain integrity and regulatory compliance. Each step—from assessing current processes and selecting the appropriate technology to training staff and regularly monitoring system performance—demonstrates how these actions collectively enhance operational efficiency, mitigate risks associated with counterfeit products, and comply with industry regulations such as the Drug Supply Chain Security Act (DSCSA). This comprehensive approach not only safeguards the supply chain but also reinforces the commitment to quality and reliability in pharmaceutical logistics.
Introduction
The pharmaceutical industry is engaged in a relentless battle against counterfeit medications, underscoring the critical need for robust track and trace systems. These systems not only enhance the integrity of the supply chain but also ensure compliance with stringent regulations such as the Drug Supply Chain Security Act (DSCSA). The challenge, however, lies in effectively establishing a track and trace system that meets both operational requirements and regulatory demands.
How can pharmaceutical companies navigate this complex landscape to protect public health while optimizing their packaging processes? This question is pivotal as the industry strives for solutions that ensure safety and compliance.
Understand the Importance of Track and Trace Systems in Pharma Packaging
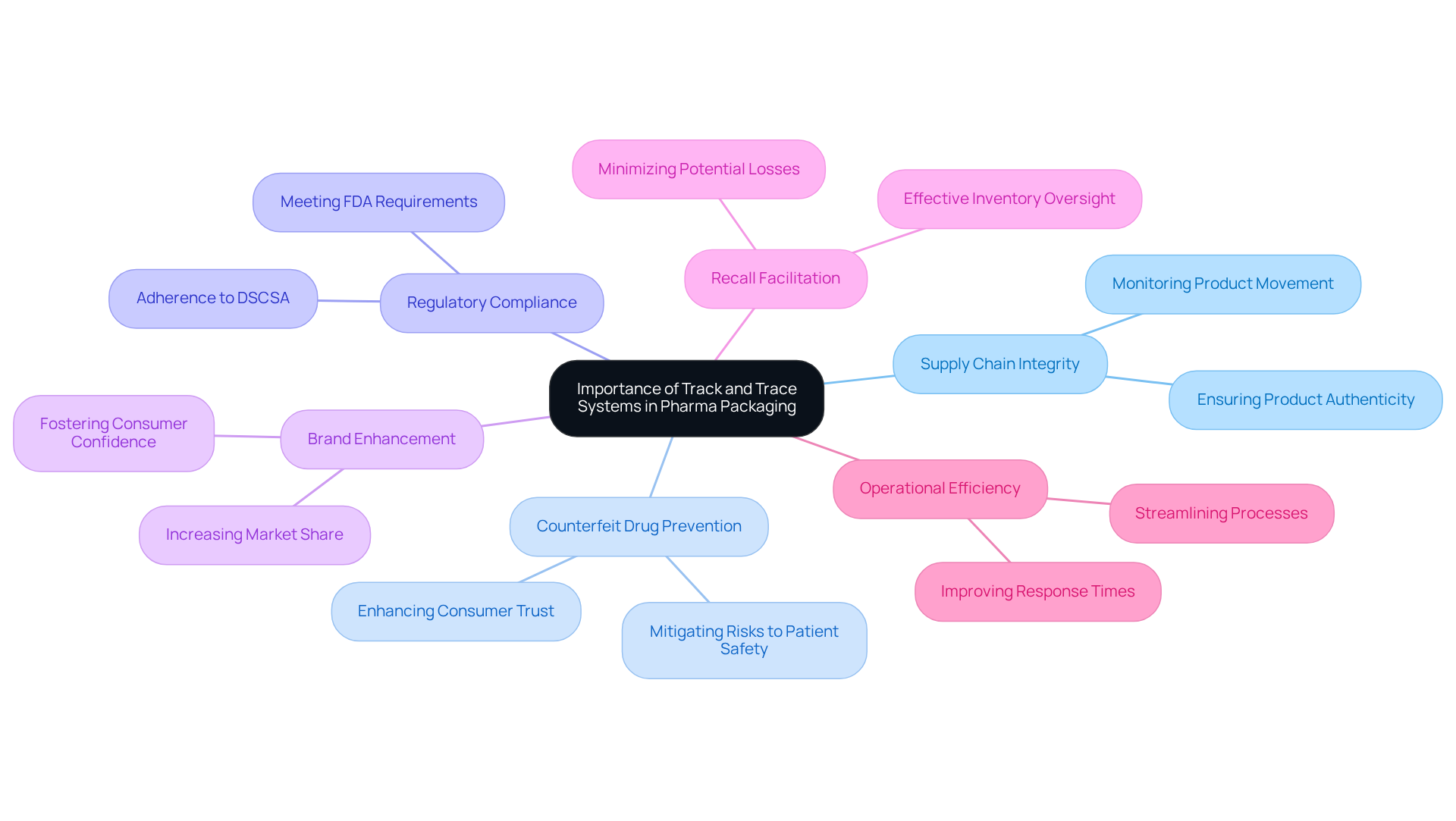
The track and trace system for pharma packaging is essential in the pharmaceutical industry for several compelling reasons. They ensure the integrity of the supply chain through a track and trace system for pharma packaging, allowing manufacturers to monitor the movement of products from production to distribution. This capability is crucial in the fight against counterfeit medications, and implementing a track and trace system for pharma packaging is essential to mitigate the significant risks to patient safety.
Furthermore, regulatory agencies, such as the FDA, mandate adherence to frameworks like the Drug Supply Chain Security Act (DSCSA), which requires a track and trace system for pharma packaging. By establishing a robust monitoring and identification framework, businesses can enhance their brand image and foster consumer confidence, ultimately leading to a larger market share. Additionally, these frameworks facilitate effective recalls and inventory oversight, minimizing potential losses and improving operational efficiency.
To effectively establish a monitoring and identification framework, stakeholders must first recognize the importance of a track and trace system for pharma packaging in safeguarding public health and ensuring compliance with industry regulations. This understanding will inform the subsequent steps in the implementation process.
Assess Current Packaging Processes and Identify Needs
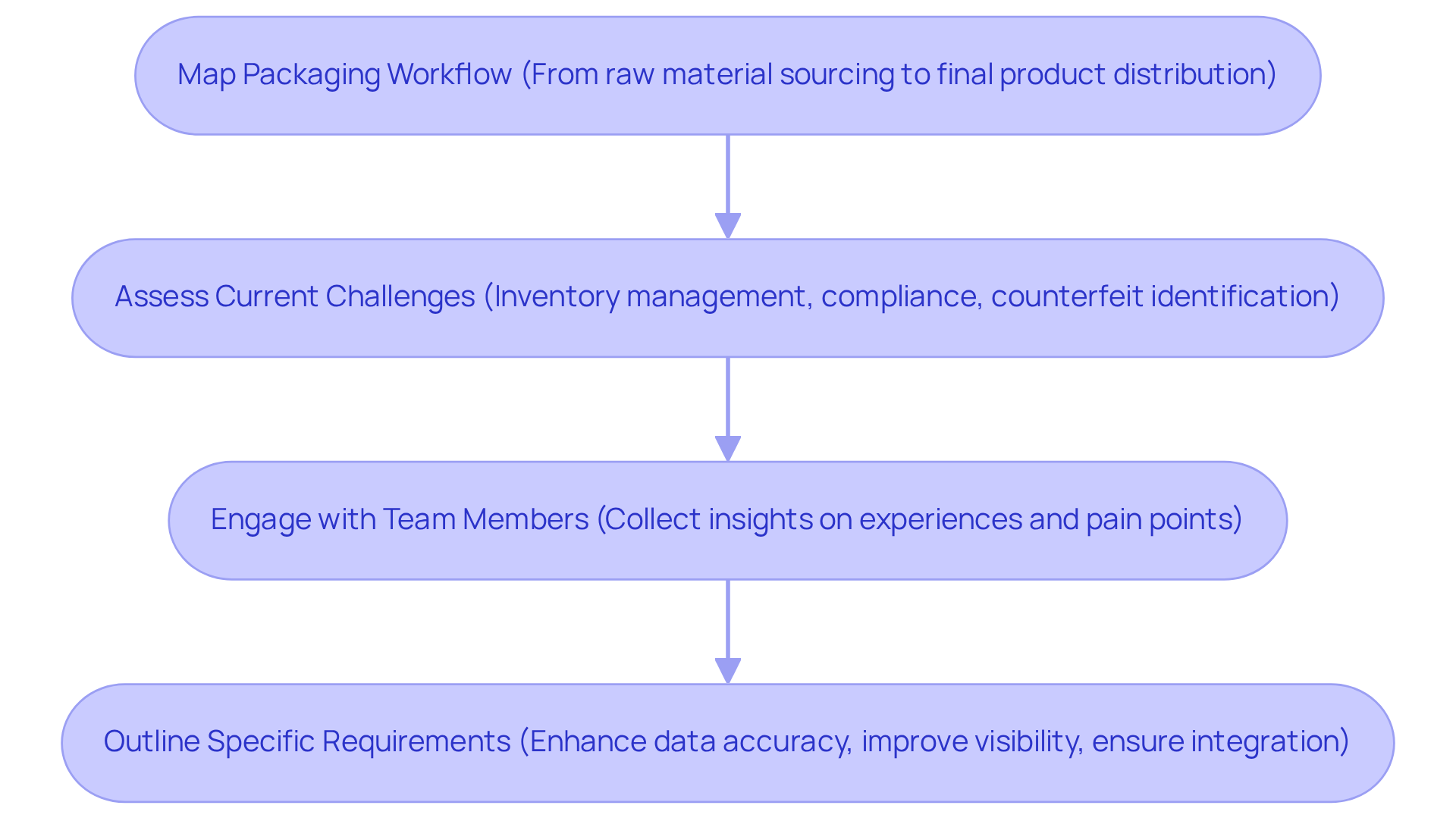
To begin establishing a robust monitoring and identification approach, conduct a comprehensive evaluation of your existing packaging procedures. Start by meticulously mapping out the entire packaging workflow, from raw material sourcing to final product distribution. Identify key stakeholders involved at each step and gather data on current technologies and practices.
Next, assess the current challenges faced in the track and trace system for pharma packaging. This may encompass issues related to inventory management, compliance with regulations, or difficulties in identifying counterfeit products using a track and trace system for pharma packaging. Engage with team members across departments to collect insights on their experiences and pain points.
Once you have a clear understanding of the existing procedures and challenges, outline the specific requirements that the new monitoring and identification solution must address. This could involve enhancing data accuracy, improving real-time visibility, or ensuring seamless integration with existing software solutions. By recognizing these needs in advance, you can ensure that the chosen solution aligns with your operational objectives and enhances overall efficiency.
Select Technology and Software for Track and Trace Implementation
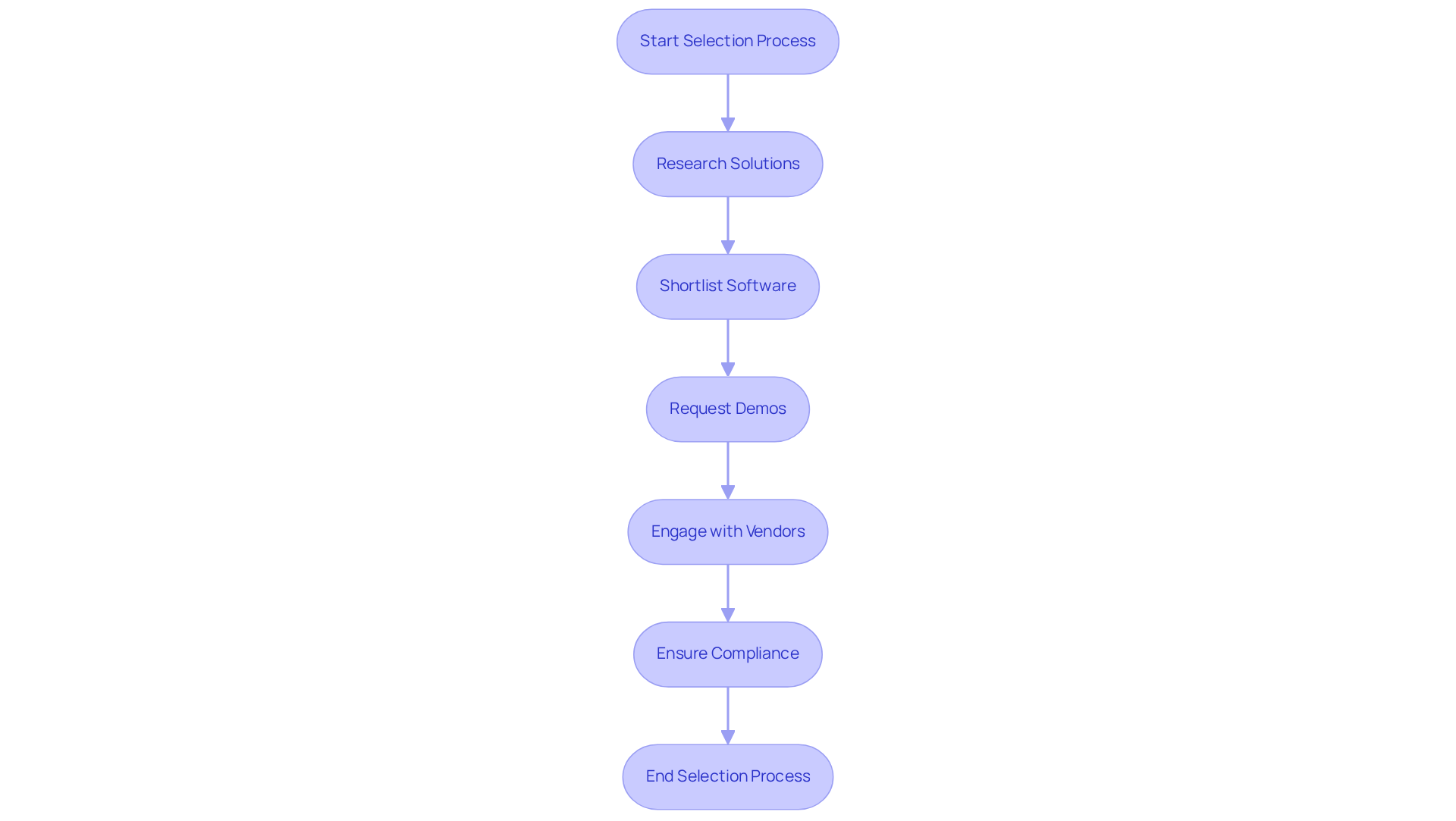
Selecting the appropriate technology and software for your track and trace system for pharma packaging is a critical step in the implementation phase, especially with the impending compliance deadlines set by the FDA for the Drug Supply Chain Security Act (DSCSA). Manufacturers and Repackagers are required to comply by May 27, 2025, while Wholesale Distributors must meet the deadline by August 27, 2025. Begin by conducting thorough research on various solutions available in the market, focusing on those that incorporate a track and trace system for pharma packaging specifically tailored for the pharmaceutical industry. Key features to consider include:
- Serialization capabilities: Ensure the software can generate unique identifiers for each product, which is essential for effective tracking.
- Integration options: Look for platforms that can seamlessly connect with your existing ERP or inventory management software.
- User-friendliness: The interface should be intuitive to facilitate easy adoption by staff members.
- Compliance features: Verify that the software adheres to regulatory requirements, such as those outlined by the DSCSA, which are essential for a track and trace system for pharma packaging.
Chester 'Chip' Davis, Jr., president and CEO of the Healthcare Distribution Alliance (HDA), underscores the significance of aligning data exchange processes among supply chain partners to achieve comprehensive DSCSA implementation, which is vital for an effective track and trace system for pharma packaging. After shortlisting potential solutions, request demos or trials to assess their functionality in real-world scenarios. Engage with vendors to discuss your specific needs and seek their recommendations based on your operational requirements. Additionally, consider the insights from the case study on the 'Creation of Electronic Monitoring and Surveillance Network,' which illustrates the practical benefits of implementing monitoring and surveillance solutions and the advantages of complying with the DSCSA. This meticulous selection process will ensure that you choose a technology, specifically a , that not only addresses your current needs but also scales with your business as it evolves, particularly in light of the pricing pressures and stricter regulatory requirements facing the pharmaceutical industry.
Train Staff on Track and Trace System Operations
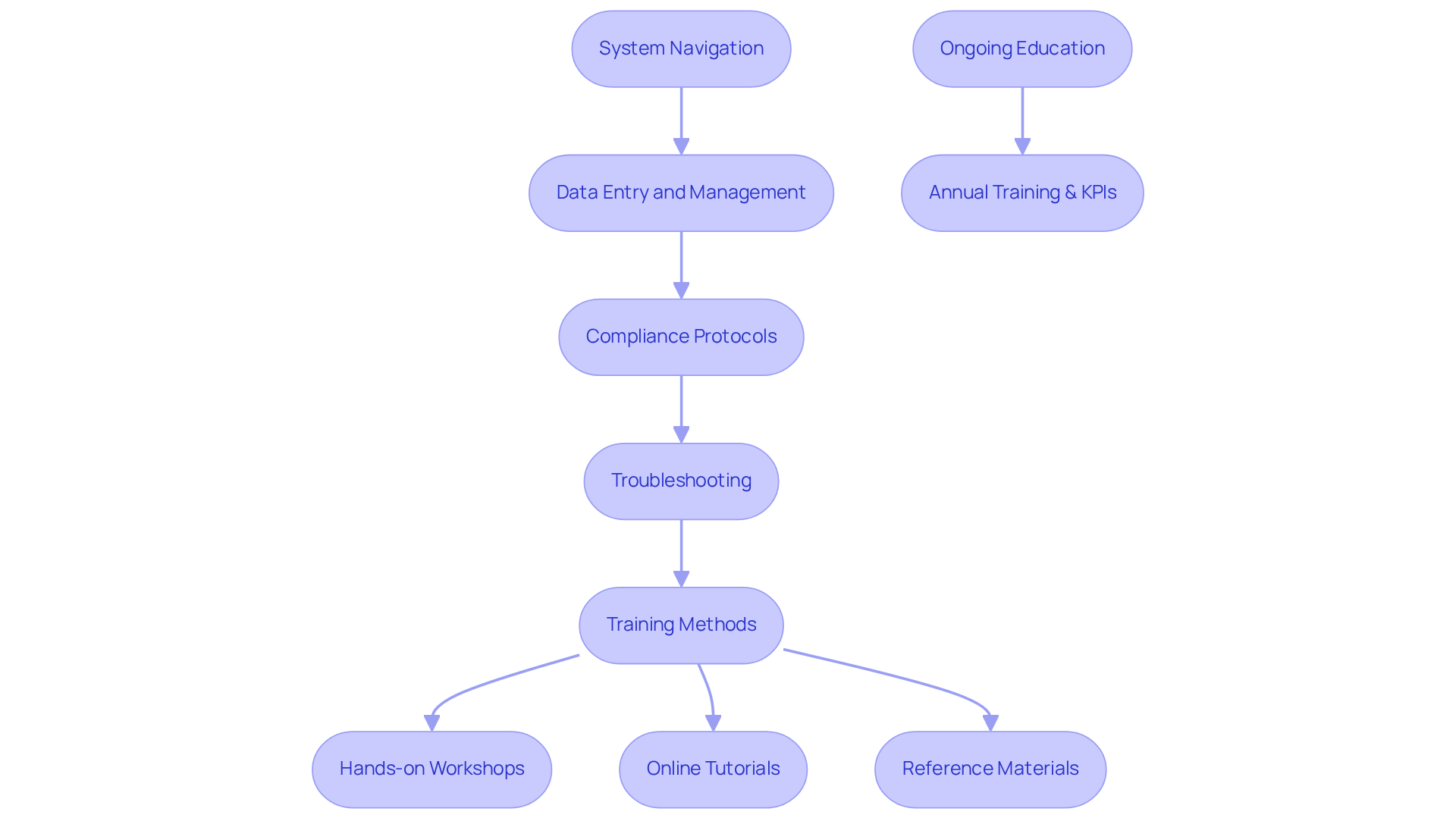
Once the track and trace system for pharma packaging is chosen and implemented, educating your personnel on its functions becomes essential. A well-structured training program should encompass all critical aspects of the system, including:
- System navigation: Instruct employees on how to effectively navigate the software interface and utilize essential features.
- Data entry and management: Provide clear guidelines for accurate data input and management to ensure traceability and compliance.
- Compliance protocols: Educate personnel on the regulatory requirements associated with the track and trace system for pharma packaging, emphasizing the importance of following these standards, including good practice (GxP) training relevant to laboratory, manufacturing, and clinical practices.
- Troubleshooting: Equip employees with the skills necessary to identify and resolve common issues that may occur during operation.
Employ a blend of training methods, such as hands-on workshops, online tutorials, and comprehensive reference materials, to accommodate various learning styles. Regular should be provided to keep staff updated about improvements and evolving regulatory requirements. Given that pharma employees should receive training at least annually, this ongoing education is crucial for maintaining compliance and operational efficiency. Furthermore, setting benchmarks and KPIs to assess training effectiveness will guarantee that the program meets its objectives and aligns with organizational goals. Investing in comprehensive training not only enables your team to optimize the functionalities of the track and trace system for pharma packaging but also greatly improves operational efficiency and compliance, ultimately protecting your organization from possible regulatory challenges.
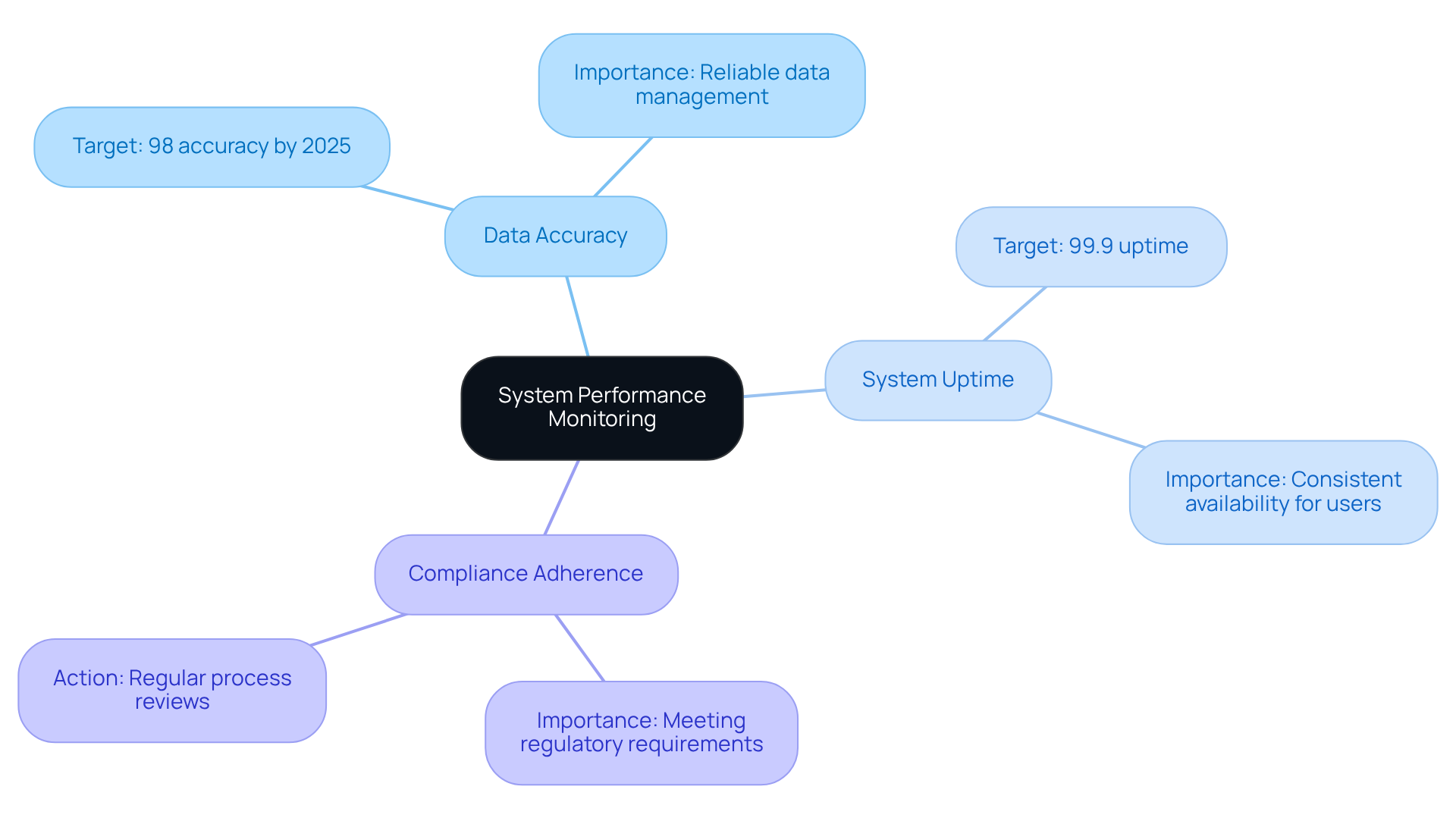
Monitor and Evaluate System Performance Regularly
To guarantee the long-term success of your monitoring and identification setup, consistent assessment and evaluation of its performance are essential. Establish key performance indicators (KPIs) that align with your operational goals, including:
- Data Accuracy: Track the percentage of accurate entries in the system, which is vital for identifying training needs and ensuring reliable data management. In 2025, data accuracy rates in the track and trace system for pharma packaging are expected to surpass 98%, highlighting the significance of precision in pharmaceutical operations. As Peter Drucker famously stated, "What gets measured gets managed," underscoring the necessity of measurement in performance evaluation.
- System Uptime: Measure the reliability of the software to guarantee consistent availability for users. Striving for an uptime of 99.9% can greatly improve operational efficiency.
- Compliance Adherence: Regularly review processes to ensure they meet evolving regulatory requirements, which are critical for maintaining market access and avoiding penalties.
Conduct periodic audits to assess the effectiveness of the system and gather feedback from staff regarding their experiences. This feedback is invaluable for pinpointing areas needing improvement and for making necessary adjustments to workflows or training programs. Additionally, staying informed about industry advancements and regulatory changes is essential for adapting your track and trace system for pharma packaging processes. It is also important to reassess KPIs when objectives are completed to confirm they remain relevant and effective. By committing to ongoing evaluation, you can ensure your system meets evolving needs and maintains a high standard of operational excellence. Incorporating case studies that illustrate can further enhance understanding and application of these principles.
Conclusion
Implementing a track and trace system for pharmaceutical packaging transcends mere regulatory compliance; it is a fundamental element in safeguarding the safety and integrity of the supply chain. By establishing such a system, pharmaceutical companies can effectively combat counterfeit products, enhance operational efficiency, and ensure adherence to stringent regulations. The journey toward implementing this system necessitates a comprehensive understanding of its significance, careful assessment of current processes, selection of the appropriate technology, thorough staff training, and ongoing performance evaluation.
Key steps have been outlined throughout this article to facilitate the successful implementation of a track and trace system. These steps include:
- Assessing existing packaging processes to identify specific needs
- Selecting technology that meets regulatory compliance
- Training staff to adeptly navigate the new system
- Regularly monitoring system performance through established key performance indicators
Each of these steps contributes to a robust framework that not only protects public health but also bolsters consumer confidence and enhances brand reputation.
As the pharmaceutical industry continues to evolve, the importance of implementing effective track and trace systems cannot be overstated. Organizations are urged to take proactive measures in adopting these systems, ensuring they remain ahead of regulatory deadlines and protect their operations against potential risks. By committing to a comprehensive track and trace strategy, pharmaceutical companies will not only safeguard their products but also contribute to a safer healthcare environment for all.
Frequently Asked Questions
Why are track and trace systems important in pharma packaging?
Track and trace systems are crucial in pharma packaging as they ensure the integrity of the supply chain, help combat counterfeit medications, and are essential for patient safety. They also comply with regulatory requirements, such as the Drug Supply Chain Security Act (DSCSA).
What regulatory requirements exist for track and trace systems in the pharmaceutical industry?
Regulatory agencies like the FDA mandate adherence to frameworks such as the Drug Supply Chain Security Act (DSCSA), which requires the implementation of track and trace systems in pharma packaging.
How can track and trace systems enhance a company's brand image?
By establishing a robust monitoring and identification framework, businesses can enhance their brand image and foster consumer confidence, which can lead to a larger market share.
What benefits do track and trace systems provide in terms of operational efficiency?
Track and trace systems facilitate effective recalls and inventory oversight, which minimizes potential losses and improves operational efficiency.
What is the first step in establishing a monitoring and identification framework for pharma packaging?
The first step is to conduct a comprehensive evaluation of existing packaging procedures, mapping out the entire workflow from raw material sourcing to final product distribution.
How can organizations identify challenges in their current track and trace systems?
Organizations can identify challenges by engaging with team members across departments to gather insights on their experiences, as well as assessing issues related to inventory management, compliance, and counterfeit product identification.
What should organizations outline after assessing their current packaging processes?
Organizations should outline the specific requirements that the new monitoring and identification solution must address, such as enhancing data accuracy, improving real-time visibility, and ensuring seamless integration with existing software solutions.




