Overview
The article delineates four pivotal strategies for achieving effective drug packaging compliance:
- Quality
- Serialization
- Integrated solutions
- Third-party logistics
Each strategy is underpinned by compelling examples and robust evidence that illustrate how they bolster product security, streamline operations, and ensure adherence to regulatory standards. Ultimately, these strategies play a crucial role in safeguarding pharmaceutical integrity.
Introduction
The integrity of pharmaceutical products is fundamentally linked to effective drug packaging compliance, a critical factor that transcends mere aesthetics. As regulatory bodies impose stricter quality standards, manufacturers encounter the dual challenge of ensuring product safety while navigating a complex compliance landscape. This article explores four key strategies that not only enhance packaging quality but also strengthen compliance efforts, ultimately safeguarding patient safety.
What innovative approaches can companies adopt to stay ahead in this dynamic regulatory environment?
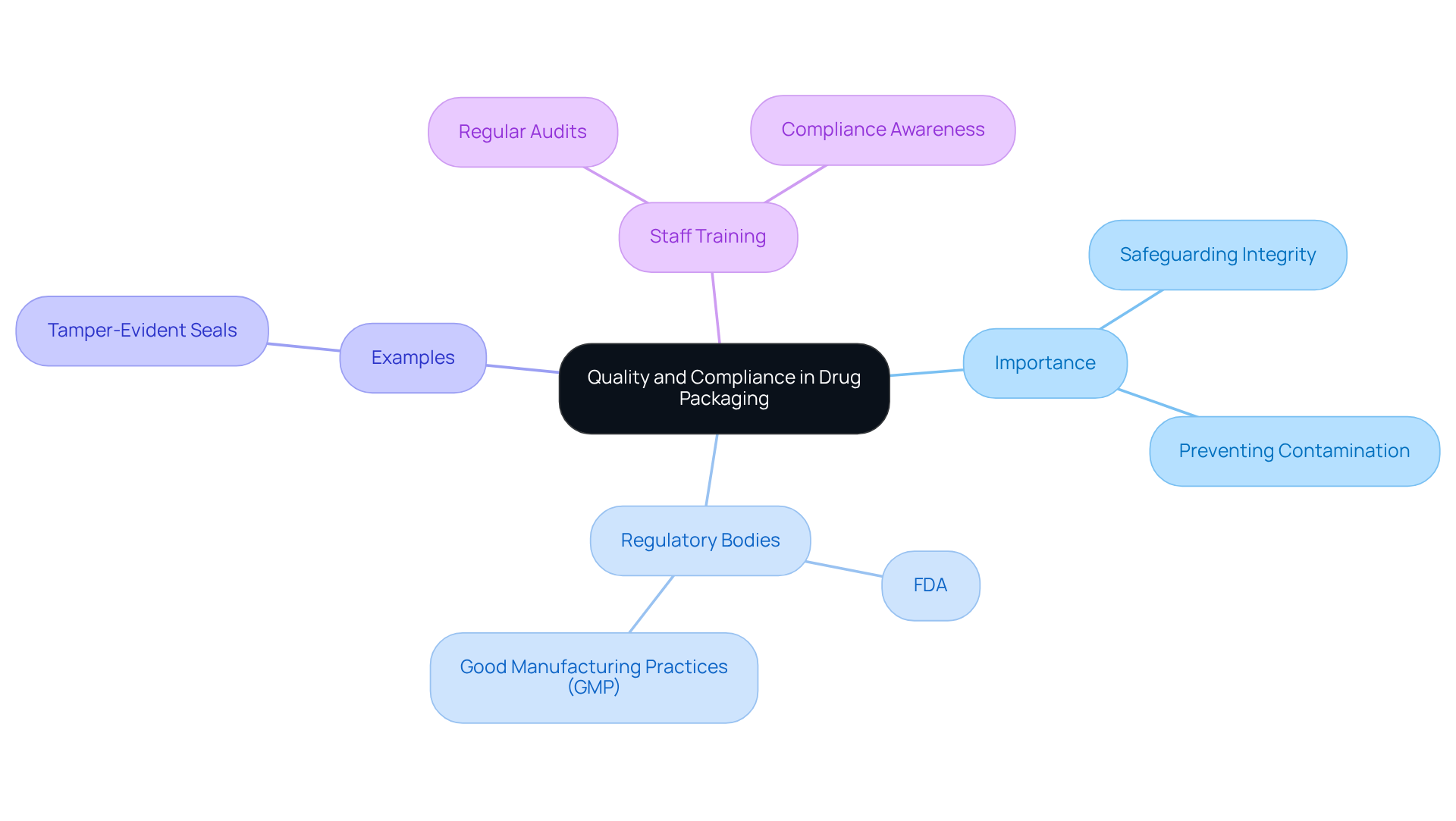
Understand the Importance of Quality and Compliance in Drug Packaging
Quality and compliance in drug presentation are paramount for safeguarding the integrity of pharmaceutical products. Drug packaging serves as the first line of defense against contamination, degradation, and counterfeiting. Regulatory bodies, such as the FDA, enforce stringent guidelines that require manufacturers to adhere to Good Manufacturing Practices (GMP). This includes ensuring that drug packaging materials are suitable for their intended use and that they maintain the product's efficacy throughout its shelf life.
For instance, using tamper-evident seals not only complies with regulations but also enhances consumer trust. Companies should conduct regular audits and training sessions to ensure that all staff are aware of compliance requirements and the importance of quality control in packaging processes.
Implement Serialization to Enhance Product Security and Compliance
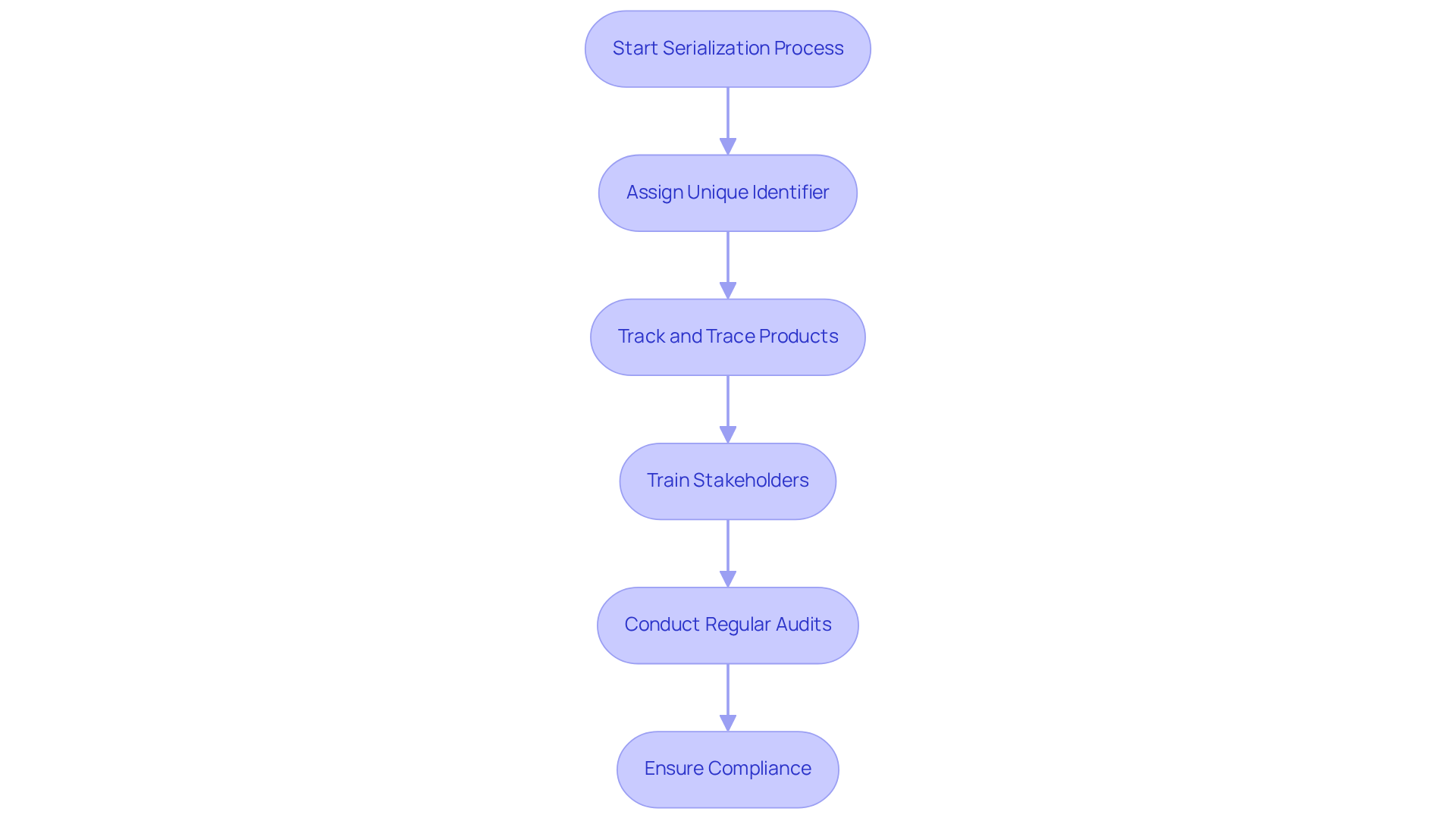
Serialization is a critical process that involves assigning a unique identifier to each saleable unit of a drug. This is essential for tracking and tracing products throughout the supply chain. By implementing serialization, organizations can significantly reduce the risk of counterfeit drugs entering the market and streamline the recall process when necessary.
For example, a drug manufacturer that embraced serialization reported a remarkable 30% decrease in instances of counterfeit products.
To successfully implement serialization, it is imperative that organizations invest in robust tracking systems and provide comprehensive training for all stakeholders within the supply chain regarding the importance of serialization. Additionally, conducting regular audits is essential to ensure compliance with serialization requirements, reinforcing the reliability of the entire system.
Utilize Integrated Packaging Solutions for Streamlined Operations
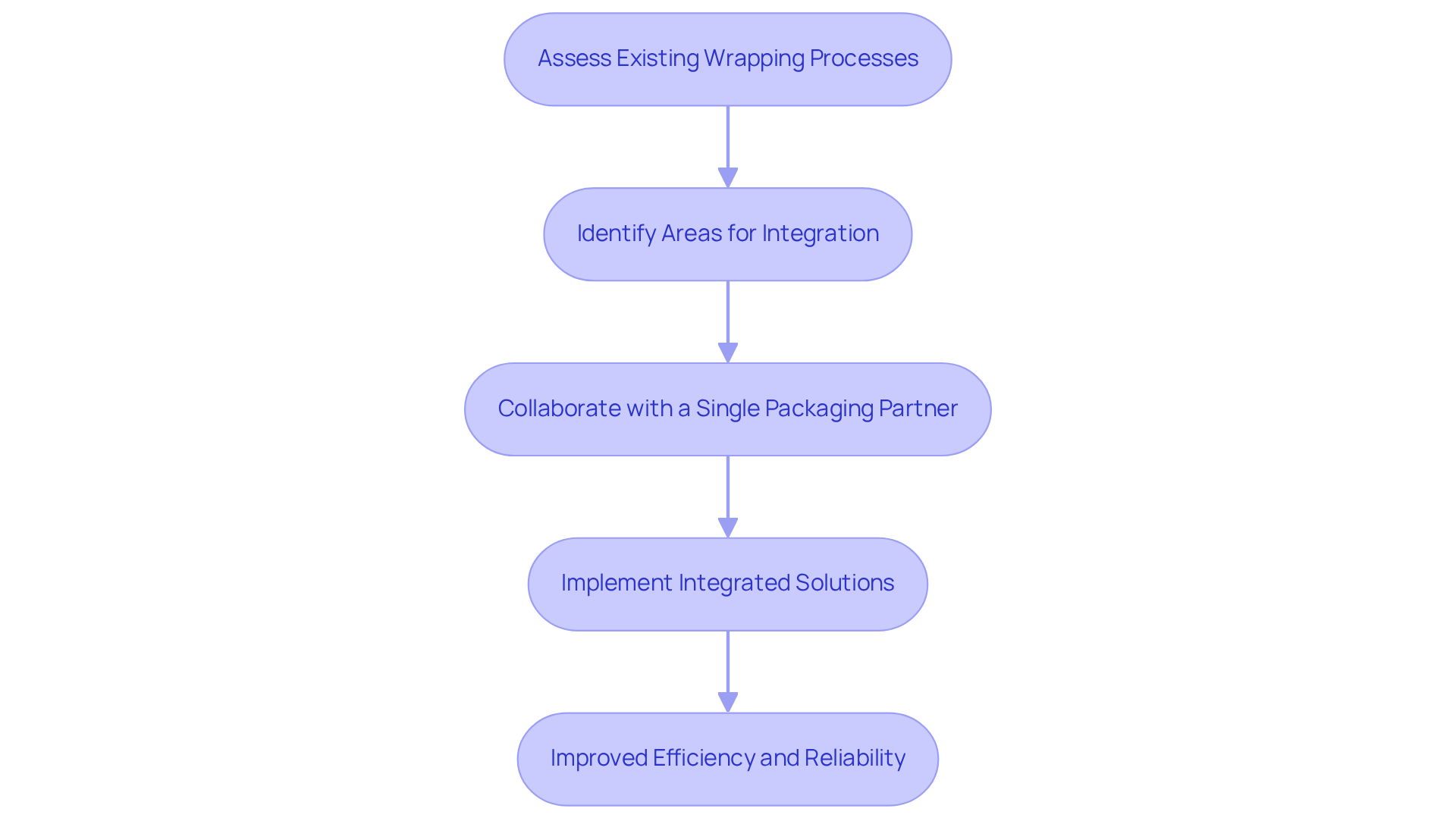
Employing integrated drug packaging solutions allows pharmaceutical firms to simplify their operations by consolidating design, filling, and logistics within a single location. This strategic approach not only mitigates the complexities associated with managing multiple vendors but also throughout the supply chain. A notable case is that of a company that implemented a unified solution, reporting a 25% reduction in time-to-market for new products.
To successfully execute this strategy, companies must assess their existing wrapping processes and identify areas where integration can yield efficiency improvements. Collaborating with a single drug packaging partner can significantly streamline operations and enhance quality control, ensuring reliability in the delivery of pharmaceutical products.
Leverage Third-Party Logistics for Efficient Distribution Management
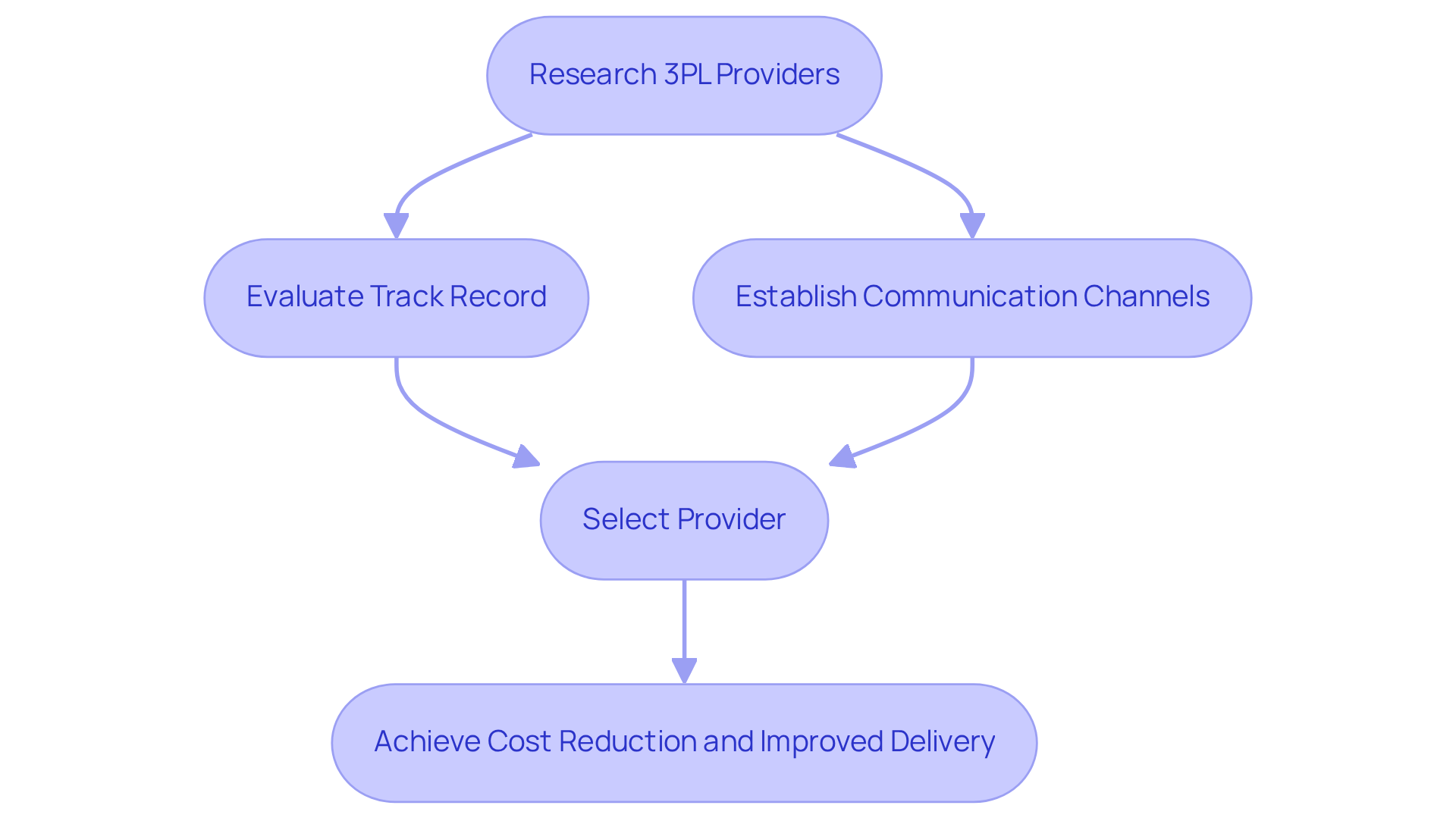
External service providers present a diverse array of solutions that significantly enhance the effectiveness of drug distribution. By outsourcing supply chain operations, companies can reduce expenses, improve scalability, and ensure compliance with regulatory requirements.
For example, a drug manufacturer that partnered with a third-party logistics provider (3PL) achieved a remarkable 40% reduction in logistics costs while simultaneously improving delivery times.
To maximize the , businesses must conduct comprehensive research to select a provider with a proven track record in the pharmaceutical industry. Establishing clear communication channels and performance metrics is essential to ensure that the partnership effectively meets the company’s distribution needs.
Conclusion
Quality and compliance in drug packaging are indispensable elements that guarantee the safety and efficacy of pharmaceutical products. By adhering to stringent regulations and implementing best practices, organizations can effectively protect their products from contamination and counterfeiting, while simultaneously fostering consumer trust. Recognizing the critical nature of these components is the first step toward achieving effective drug packaging compliance.
Throughout this article, several key strategies have been underscored, including:
- The importance of serialization for tracking and tracing products
- The advantages of integrated packaging solutions for operational efficiency
- The benefits of leveraging third-party logistics for streamlined distribution management
Each of these strategies plays a vital role in enhancing compliance and safeguarding the integrity of pharmaceutical products.
In conclusion, the importance of quality and compliance in drug packaging cannot be overstated. As the industry continues to evolve, embracing these strategies will not only enhance operational efficiency but also ensure patient safety. By prioritizing effective drug packaging compliance, organizations can significantly contribute to a more secure and trustworthy pharmaceutical landscape, ultimately benefiting consumers and manufacturers alike.
Frequently Asked Questions
Why is quality and compliance important in drug packaging?
Quality and compliance in drug packaging are crucial for safeguarding the integrity of pharmaceutical products, protecting them from contamination, degradation, and counterfeiting.
What role do regulatory bodies play in drug packaging?
Regulatory bodies, such as the FDA, enforce stringent guidelines that require manufacturers to adhere to Good Manufacturing Practices (GMP) regarding drug packaging.
What are Good Manufacturing Practices (GMP)?
Good Manufacturing Practices (GMP) are regulations that ensure drug packaging materials are suitable for their intended use and that they maintain the product's efficacy throughout its shelf life.
How do tamper-evident seals contribute to drug packaging?
Tamper-evident seals not only comply with regulations but also enhance consumer trust in the safety and integrity of the product.
What measures should companies take to ensure compliance in drug packaging?
Companies should conduct regular audits and training sessions to ensure that all staff are aware of compliance requirements and the importance of quality control in packaging processes.




