Overview
The article delineates five essential steps for effective serialization track and trace implementation in the nutraceutical sector:
- Technology selection
- Regulatory compliance
- Data management
- Pilot testing
- Staff training
These steps are vital for combating counterfeit products and ensuring product integrity. By enhancing operational efficiency and aligning with regulatory requirements, they ultimately foster consumer confidence and safety within the supply chain.
Introduction
The nutraceutical industry is currently facing an alarming rise in counterfeit products, a situation that not only jeopardizes consumer safety but also threatens the integrity of the market. Serialization and track and trace systems present a robust solution, allowing companies to assign unique identifiers to each product and monitor their journey from production to consumption.
However, the effective implementation of these systems poses significant challenges, including:
- Navigating complex regulatory requirements
- Ensuring that staff are adequately trained
How can businesses overcome these hurdles to safeguard their products and bolster consumer trust? This is a critical question that demands attention and action.
Define Serialization and Track and Trace in Nutraceuticals
The process of serialization track and trace involves assigning a unique identifier to each item unit, which enables precise tracking throughout the supply chain. Track and trace enhances this through serialization track and trace by overseeing the movement of these serialized items from production to the end consumer.
In the nutraceutical sector, these practices are essential for ensuring authenticity, which is increasingly crucial as the industry faces significant challenges from counterfeit items—estimated to cost the pharmaceutical sector around $40 billion annually.
The global track and trace solutions market was valued at USD 4.1 billion in 2022 and is projected to reach USD 14.3 billion by 2030, growing at a CAGR of 19.3% from 2023 to 2030.
By employing strong data encoding and serialization track and trace systems, companies can effectively manage product recalls, reduce counterfeit risks, and enhance transparency within their supply chains. This not only encourages consumer confidence but also guarantees adherence to changing regulatory criteria, establishing in the nutraceutical supply chain.
Furthermore, leveraging high-quality data and advanced technologies like AI can significantly improve inspection processes, reduce false rejects, and support overall operational efficiency.
![]()
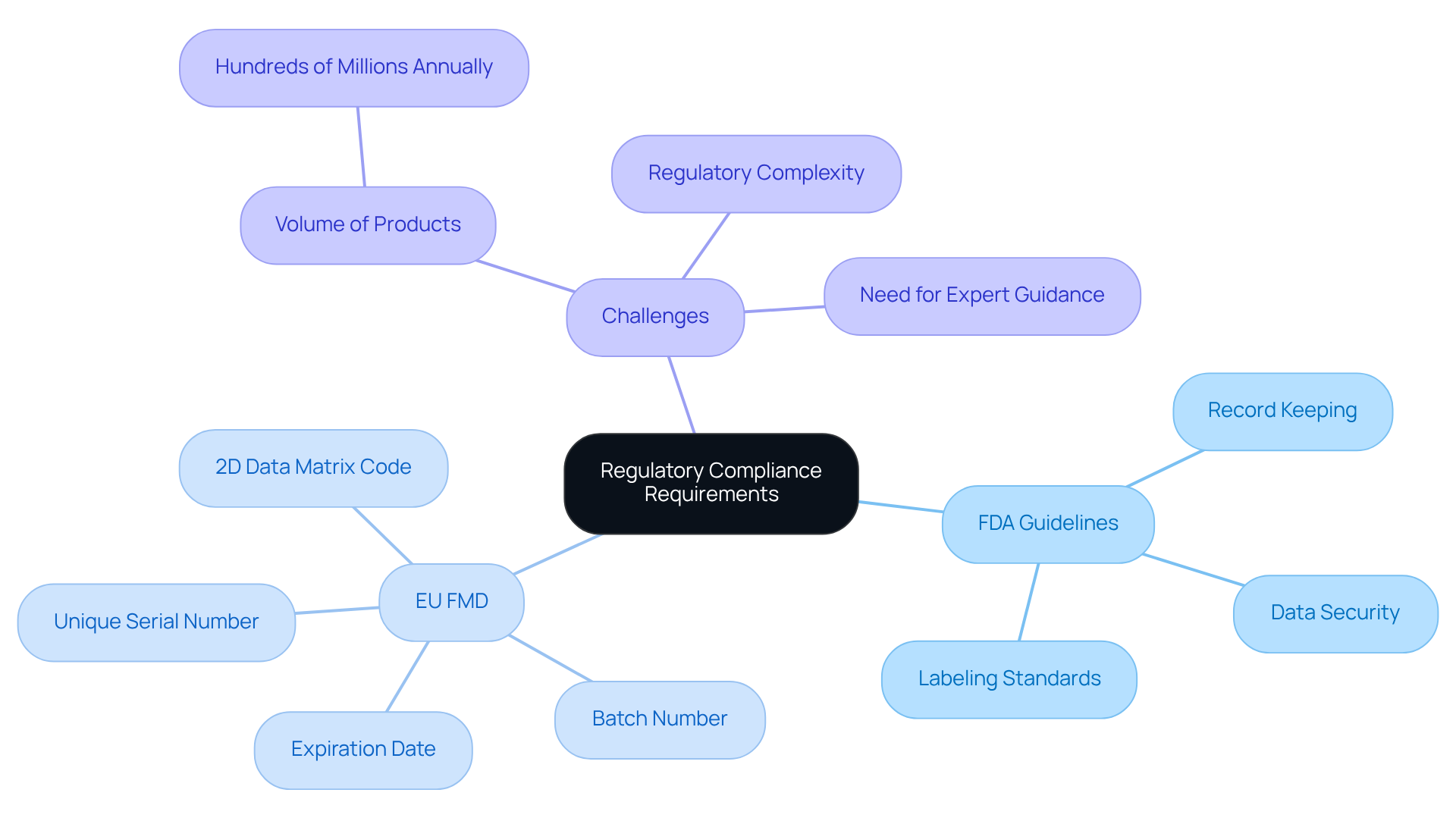
Identify Regulatory Compliance Requirements
To successfully implement serialization track and trace, it is crucial to identify the relevant regulatory compliance requirements. This includes understanding guidelines set forth by organizations such as the FDA and international standards like the , which has been in effect since February 9, 2019. Key requirements often involve:
- Maintaining detailed records of item movement for serialization track and trace
- Ensuring that serialized data is accessible and secure
- Adhering to specific labeling standards
For instance, the FMD mandates that each unit of drug must feature a unique serial number, batch number, and expiration date encoded in a 2D data matrix code.
Pharmaceutical companies face significant challenges in meeting these requirements, particularly given the volume of products that must undergo serialization track and trace, often reaching hundreds of millions of units annually. Interacting with regulatory specialists can offer valuable insights into navigating these complex regulations. Firms such as Merck and Ferrer have effectively executed strategies to guarantee adherence to the FMD, highlighting the significance of early planning and a network-focused method to handling extensive collaboration among various partners and frameworks. By performing a comprehensive examination of relevant regulations and utilizing expert advice, businesses can successfully align their operations with the changing environment of product identification and tracking requirements.
Implement Serialization and Track and Trace Systems
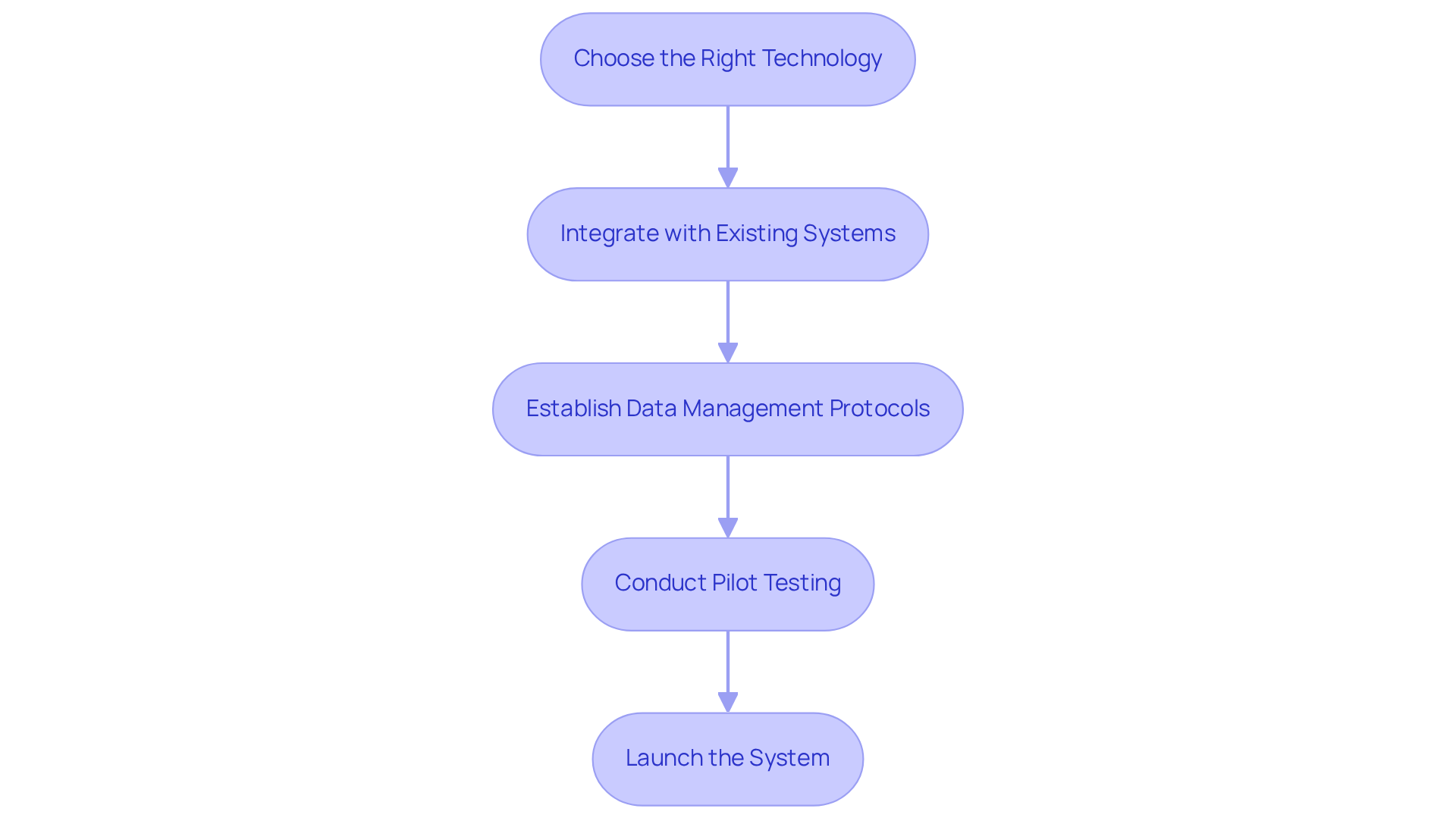
Implementing serialization and track-and-trace systems involves several key steps:
- Choose the Right Technology: Selecting the appropriate software and hardware solutions is critical to meet serialization and track-and-trace requirements. This includes barcode scanners, RFID technologies, and cloud-based software for effective data management. Notably, RFID technologies have shown marked improvements in tracking precision and speed compared to conventional barcode methods. Research indicates that RFID can enhance tracking accuracy by as much as 99% and reduce processing time by 30%. Consequently, these technologies are increasingly favored by organizations.
- Integrate with Existing Systems: It is essential to ensure that the new data formatting system integrates seamlessly with current production and inventory management systems. This integration is vital for maintaining operational efficiency and minimizing disruptions. For instance, organizations can utilize APIs to connect data processing software with existing inventory systems, facilitating real-time information exchange. As Liz Cornish, a senior digital marketing & brand manager, aptly states, "Integrating AI into serialization not only improves adherence and anti-counterfeiting efforts but also fosters deeper insights into supply chains."
- Establish Data Management Protocols: Robust protocols for data entry, storage, and retrieval must be created to maintain accurate records of serialized products. This involves defining roles and responsibilities for data management and ensuring compliance with regulatory standards. Effective data management is paramount for ensuring serialization track and trace, as well as accountability throughout the supply chain. The FDA's new guidance, which includes a stabilization period from November 2023 to November 2024 for new tracking requirements, underscores the importance of adhering to these protocols.
- Conduct Pilot Testing: Prior to full-scale implementation, conducting pilot tests is crucial to identify potential issues and refine processes. This step allows organizations to evaluate the in a controlled environment, ensuring that any challenges are addressed before broader implementation.
- Launch the System: Once testing is complete, the system should be rolled out across all relevant operations. It is imperative to ensure that all personnel are trained on the new protocols and understand the significance of tracking in preventing counterfeiting and maintaining integrity. Involving employees in the implementation process fosters a culture of adherence and accountability.
By following these steps, nutraceutical producers can effectively establish tracking and tracing methods, thereby enhancing safety and regulatory compliance.
Train Staff on Serialization and Track and Trace Procedures
Training personnel on is vital for ensuring adherence and operational efficiency.
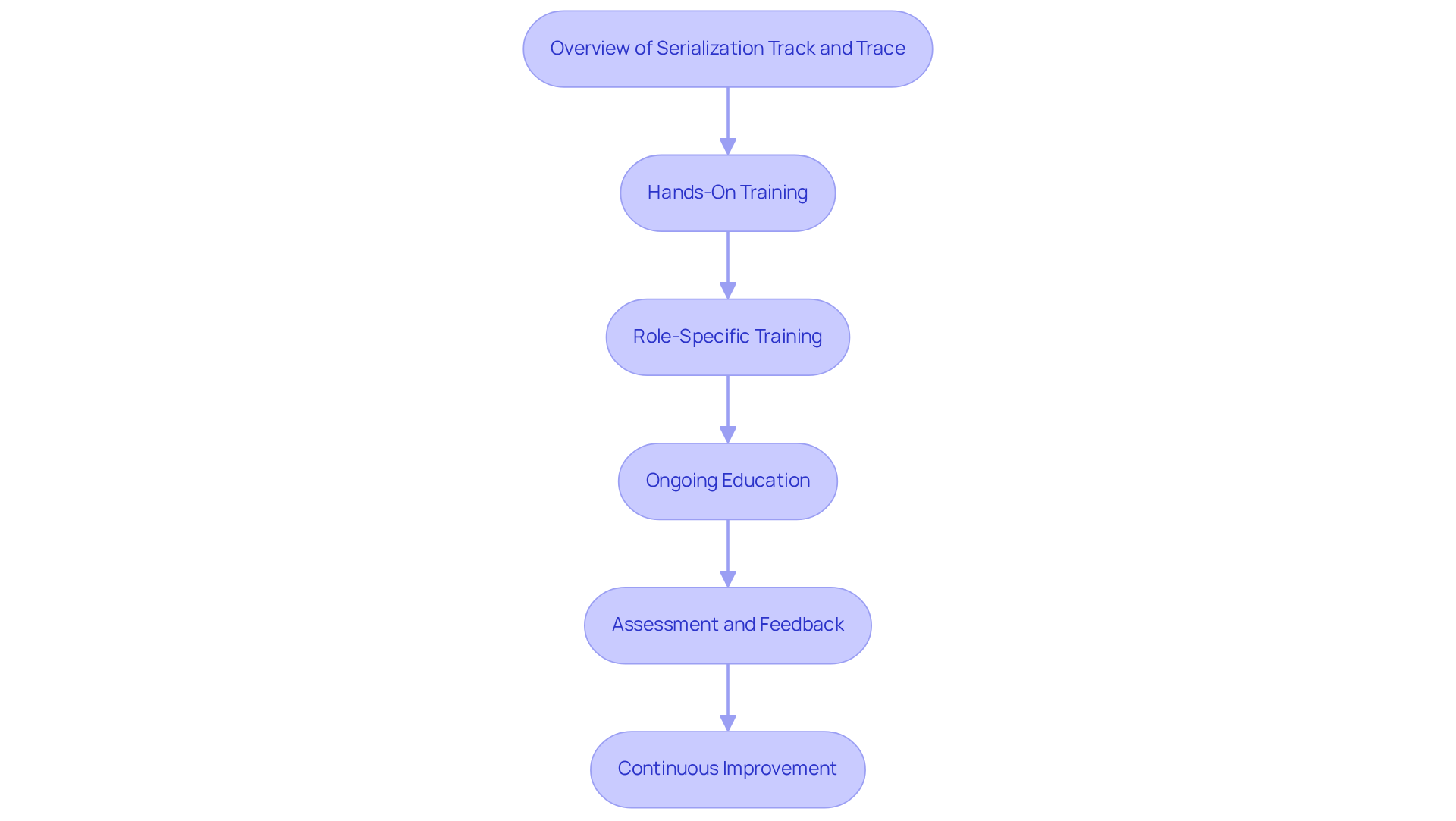
Overview of Serialization Track and Trace:
Establish a foundational understanding of the importance of these frameworks in combating counterfeiting and ensuring product integrity. As Patrick Lemay states, 'Serialization track and trace solutions assist manufacturers in streamlining the documentation of materials, personnel, and processes involved in production, automatically generating a digital audit trail, and alleviating the burden of regulatory compliance for manufacturers.'
Hands-On Training:
Facilitate practical sessions where employees interact directly with tracking technology and software, enhancing their confidence and competence. This practical method is essential, particularly given that establishing data systems demands significant investment from pharmaceutical producers.
Role-Specific Training:
Tailor training sessions to focus on the distinct responsibilities of various roles within the organization, ensuring clarity in each employee's tasks associated with tracking.
Ongoing Education:
Develop a continuous education program that keeps staff informed about updates in procedures, technology, and regulatory requirements, fostering a culture of adaptability. This is especially significant considering regulations like the FDA's Drug Supply Chain Security Act (DSCSA) and the EU Falsified Medicines Directive (FMD), which require adherence to serialization track and trace practices.
Assessment and Feedback:
Implement regular assessments to gauge staff understanding and collect feedback to refine training programs, ensuring they meet the evolving needs of the organization. Including statistics, such as the 2.7% rise in the average cost of goods sold due to item tracking, highlights the financial consequences of efficient training and execution. This thorough approach not only improves employee skill but also bolsters the overall efficiency of tracking methods in the nutraceutical field.
Troubleshoot Common Implementation Challenges
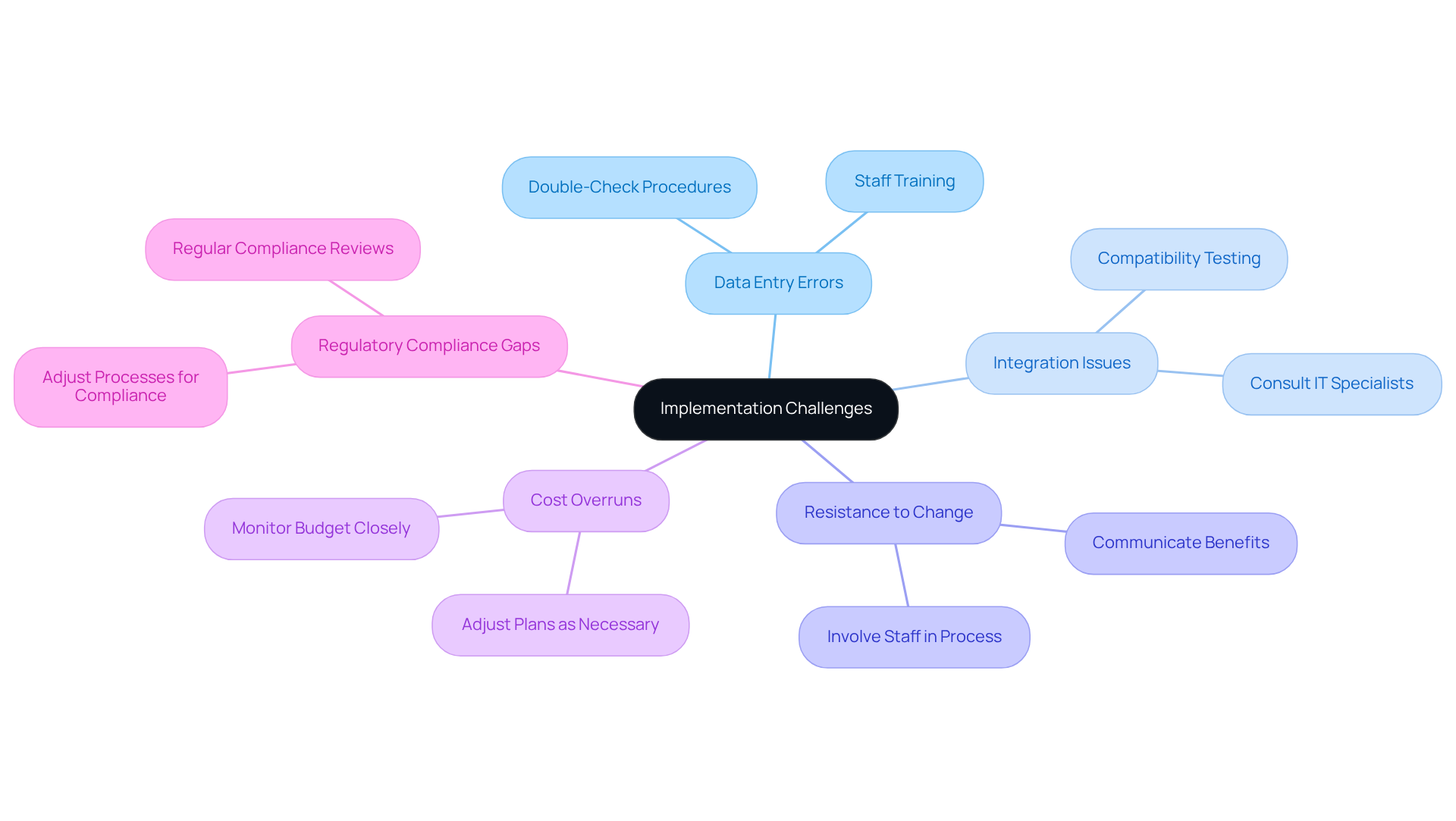
Common challenges during the implementation of serialization track and trace frameworks include:
- Data Entry Errors: It is crucial to ensure that staff are adequately trained to minimize errors during data entry. Implementing double-check procedures can effectively catch these errors.
- Integration Issues: If the new platform does not integrate seamlessly with existing software, consulting with IT specialists is advisable to resolve any compatibility concerns.
- : To address any resistance from staff, it is important to clearly communicate the benefits of the new system and actively involve them in the implementation process.
- Cost Overruns: Monitoring the budget closely is essential; adjustments to plans should be made as necessary to avoid unexpected costs.
- Regulatory Compliance Gaps: Regular reviews of compliance requirements are necessary, and processes must be adjusted to ensure ongoing adherence to regulations.
Conclusion
Implementing effective serialization and track and trace systems is vital for the nutraceutical industry. This approach not only ensures product authenticity but also enhances consumer trust and regulatory compliance. By adopting these practices, companies can combat the growing threat of counterfeiting and streamline their supply chain operations, ultimately safeguarding their reputation and financial stability.
The article outlines several key steps for successful implementation. These include:
- Selection of appropriate technologies
- Integration with existing systems
- Establishing robust data management protocols
- Conducting pilot testing
- Comprehensive staff training
Each of these steps plays a crucial role in overcoming the challenges associated with serialization and track and trace, such as data entry errors and regulatory compliance gaps. Moreover, the importance of ongoing education and adaptation in response to evolving regulations cannot be overstated.
In conclusion, the significance of serialization and track and trace in the nutraceutical sector cannot be overlooked. As the market continues to grow and regulatory demands become more stringent, companies must prioritize these systems to protect their products and foster a culture of accountability and transparency. Embracing these practices will not only mitigate risks but also enhance operational efficiency, ultimately leading to a more secure and trustworthy supply chain.
Frequently Asked Questions
What is serialization track and trace in nutraceuticals?
Serialization track and trace involves assigning a unique identifier to each item unit, allowing for precise tracking throughout the supply chain. It oversees the movement of these serialized items from production to the end consumer, ensuring authenticity and reducing counterfeit risks.
Why is serialization track and trace important in the nutraceutical sector?
It is essential for ensuring authenticity, especially as the industry faces significant challenges from counterfeit items, which cost the pharmaceutical sector around $40 billion annually. Serialization helps manage product recalls, enhances transparency, and builds consumer confidence.
What is the current market trend for track and trace solutions?
The global track and trace solutions market was valued at USD 4.1 billion in 2022 and is projected to reach USD 14.3 billion by 2030, growing at a CAGR of 19.3% from 2023 to 2030.
What are the key regulatory compliance requirements for serialization track and trace?
Key requirements include maintaining detailed records of item movement, ensuring serialized data is accessible and secure, and adhering to specific labeling standards, such as those mandated by the EU Falsified Medicines Directive (FMD).
What does the EU Falsified Medicines Directive require?
The FMD mandates that each unit of drug must feature a unique serial number, batch number, and expiration date encoded in a 2D data matrix code.
What challenges do pharmaceutical companies face in meeting serialization requirements?
Companies often face challenges due to the high volume of products that must undergo serialization track and trace, which can reach hundreds of millions of units annually.
How can companies ensure compliance with serialization regulations?
Companies can interact with regulatory specialists for insights, perform a comprehensive examination of relevant regulations, and utilize expert advice to align their operations with product identification and tracking requirements. Early planning and a collaborative approach are also crucial.




