Overview
The primary objective of this article is to deliver a comprehensive tutorial on mastering serialization within the pharmaceutical industry, emphasizing its critical importance, regulatory requirements, implementation steps, and associated benefits. This article articulates the necessity for unique identifiers to enhance traceability and ensure compliance with regulations such as the DSCSA and FMD. Furthermore, it underscores the substantial advantages of serialization in combating counterfeiting and enhancing operational efficiency throughout the supply chain.
Introduction
The pharmaceutical industry finds itself at a pivotal crossroads, where the integrity of drug supply chains faces increasing threats from counterfeiting and regulatory complexities. Serialization—assigning unique identifiers to each drug unit—emerges as a crucial strategy for enhancing traceability and compliance, promising a safer future for consumers.
However, as regulations evolve and the need for operational efficiency intensifies, pharmaceutical companies must consider how to effectively implement serialization. This implementation not only meets legal requirements but also bolsters public trust and streamlines processes, ultimately ensuring a robust and reliable supply chain.
Define Serialization in Pharmaceuticals
Serialization pharma is a critical practice in medicine that involves assigning a unique serial number to each marketable unit of a drug. This practice has become increasingly vital in today's . The unique identifier is essential for tracking and tracing items throughout the supply chain, from manufacturing to the end consumer. The worldwide pharmaceutical tracking market is projected to expand from USD 15.1 billion in 2022 to USD 24.8 billion by 2031, reflecting a compound annual growth rate (CAGR) of 5.1% from 2023 to 2031. The significance of tracking cannot be overstated.
The serial number typically encompasses essential information, including:
- The item's origin
- Batch number
- Expiration date
These details are crucial for regulatory compliance and consumer safety. Notably, Indonesia is leading the way in the ASEAN region by mandating item tracking for all prescription products by 2025, making it the first member state to enforce such a requirement. This initiative ensures that vital information is incorporated into barcodes, thereby enhancing traceability.
Moreover, the encoding process is instrumental in combating counterfeit medications. It enables each item to be verified against a secure database for authenticity—an increasingly important capability as AI-powered counterfeit schemes pose significant threats to patient safety. Additionally, the FDA has introduced a stabilization phase for new tracking requirements from November 2023 to November 2024, underscoring the evolving regulatory environment. By implementing serialization pharma, pharmaceutical companies not only comply with stringent regulations but also enhance supply chain visibility and operational efficiency, ultimately safeguarding public health.
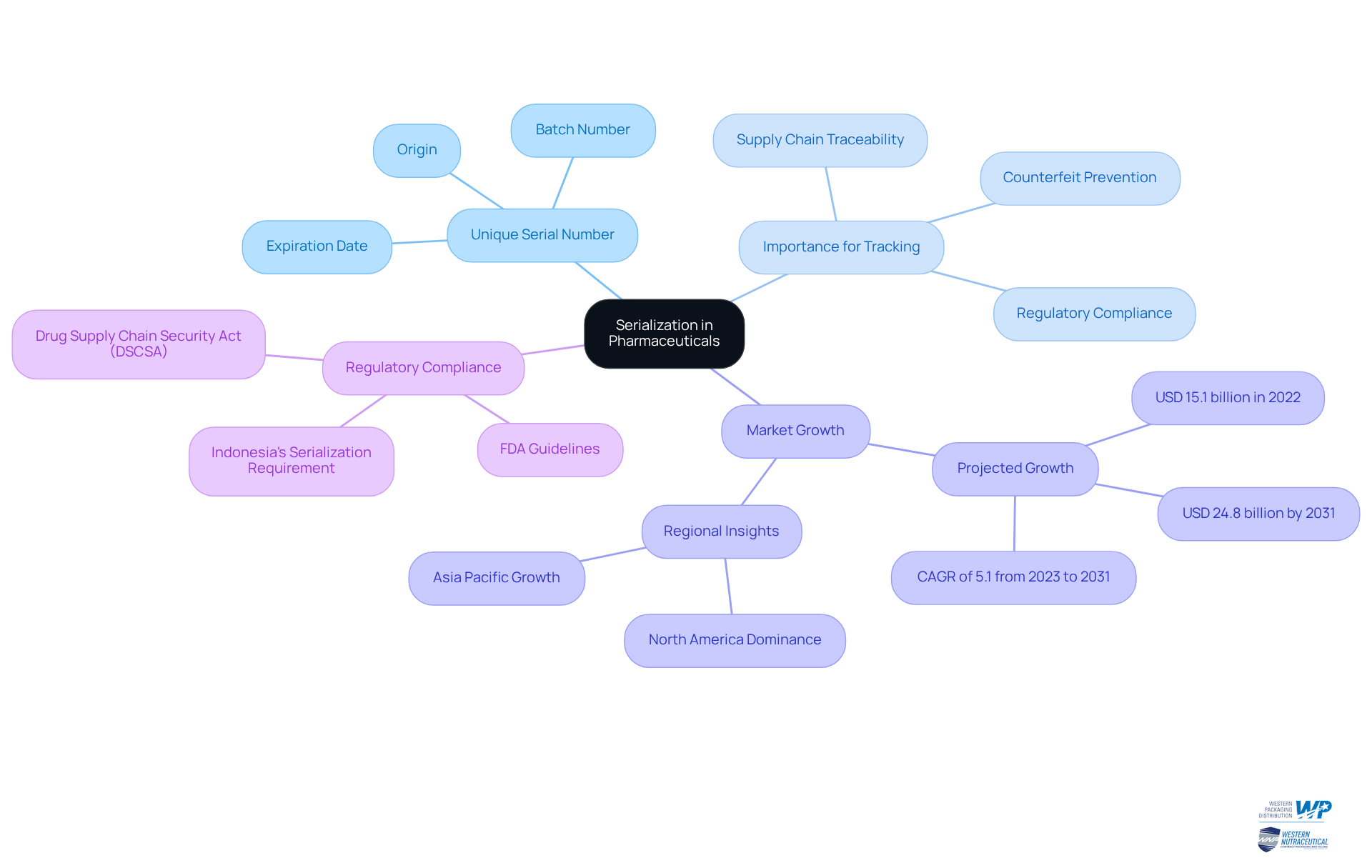
Explore Regulatory Requirements for Serialization
In the United States, the Drug Supply Chain Security Act (DSCSA) mandates that all prescription drug packages display a unique item identifier, which includes serialization pharma through a serialized National Drug Code (sNDC), by November 27, 2025. This requirement is part of a broader initiative aimed at and product traceability, which emphasizes serialization pharma, and is underscored by the enactment of the DSCSA in 2013, including a comprehensive 10-year plan for drug tracing. The requirement for full electronic tracing with verification under serialization pharma was set for November 27, 2023, emphasizing the urgency for compliance.
Similarly, the EU Falsified Medicines Directive (FMD) requires unique identification and tamper-evident features, ensuring that each package is distinctly recognized. Compliance with these regulations is not merely a legal obligation; it is essential for maintaining trust within the serialization pharma supply chain. The World Health Organization estimates that 5-8% of pharmaceuticals sold globally are counterfeit, emphasizing the critical need for serialization pharma to enhance product traceability and patient safety.
Organizations must establish robust systems for managing tracking data and reporting to regulatory authorities, particularly in serialization pharma, as non-compliance can lead to significant penalties and damage to reputation. For instance, organizations that have successfully integrated tracking processes, such as those utilizing advanced track-and-trace technologies, demonstrate the competitive advantage gained from adhering to these regulations.
Producers and repackagers must fully comply by May 27, 2025, and understanding and implementing serialization pharma tracking requirements will be vital for both producers and distributors.
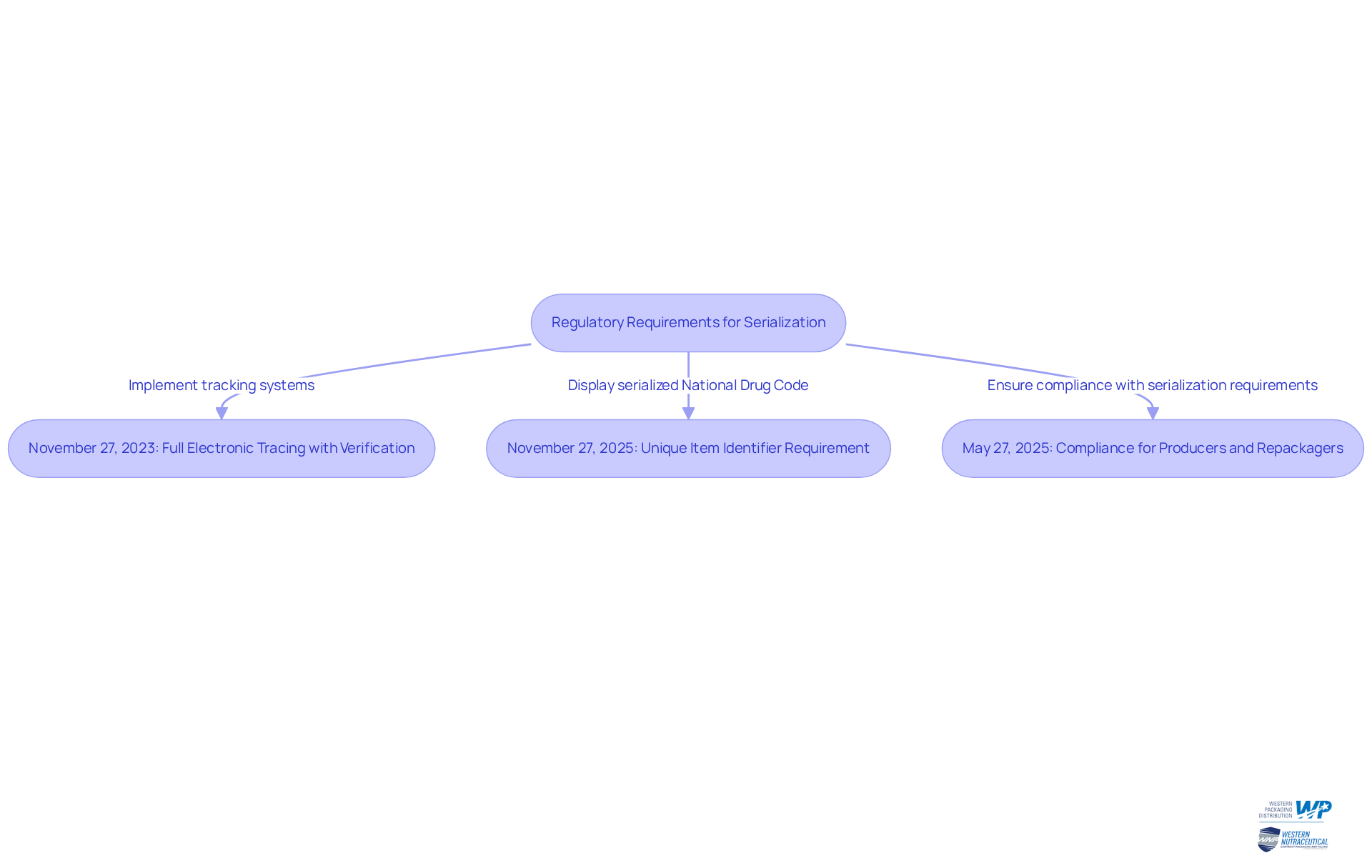
Implement Serialization in Pharmaceutical Packaging
Applying serialization pharma in pharmaceutical packaging necessitates a systematic method to guarantee compliance and efficiency. Companies must follow essential steps to achieve this:
- Assess Current Systems: Begin with a thorough review of existing packaging and labeling systems to identify deficiencies in tracking capabilities. This assessment is crucial for understanding the current state and planning necessary upgrades.
- Choose Serialization Technology: Select the most suitable technology for printing and managing serial numbers. Options include thermal inkjet and laser marking systems, recognized for their reliability and accuracy in marking.
- Integrate with Supply Chain: Ensure that tracking processes are seamlessly incorporated with supply chain management systems. This integration facilitates real-time tracking and data management, enhancing visibility throughout the supply chain.
- Train Staff: Provide extensive training for personnel on the significance of tracking and the functionality of new systems. Well-informed employees are vital for successful implementation and .
- Conduct Testing: Before large-scale execution, carry out thorough testing to ensure that the data encoding process operates smoothly and complies with all legal requirements. This step helps identify potential issues early on.
- Monitor and Adjust: After implementation, continuously oversee the data packaging process. Regular assessments allow for timely adjustments to improve efficiency and ensure compliance with evolving regulations.
According to Grand View Research, the worldwide medicine tracking market was assessed at US$ 15.1 billion in 2022 and is expected to expand at a CAGR of 5.1% from 2023 to 2031. This highlights the rising compliance requirements fueling market growth. By following these steps, drug manufacturers can effectively execute item tracking in serialization pharma, which improves traceability and compliance while addressing the increasing need for secure supply chains. The incorporation of data encoding technology not only meets regulatory standards but also significantly enhances operational efficiency, ultimately resulting in a stronger market presence.
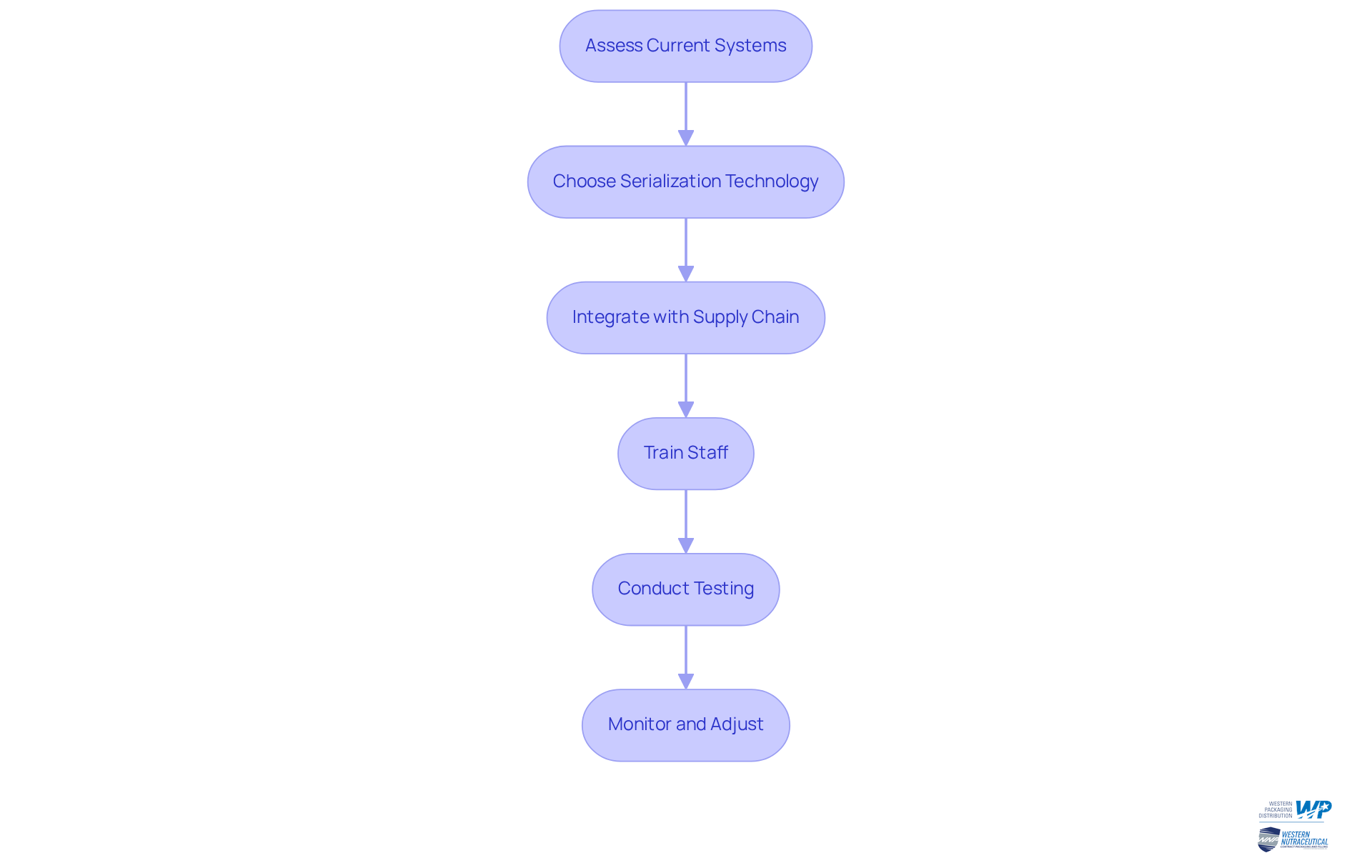
Analyze the Benefits of Serialization in Pharma
The benefits of serialization in the pharmaceutical industry are extensive and critical for ensuring product integrity and safety:
- Enhanced traceability through serialization pharma enables precise tracking of items throughout the supply chain, allowing for rapid responses to recalls or quality issues. This capability is vital in maintaining such as the U.S. Drug Supply Chain Security Act (DSCSA) and the EU's Falsified Medicines Directive (FMD), which mandate robust serialization pharma systems for traceability. Significantly, 95% of drug manufacturers in the U.S. have adopted serialization pharma tracking capabilities to adhere to the DSCSA, demonstrating the sector's dedication to traceability.
- Counterfeit prevention through serialization pharma is achieved by assigning unique identifiers to each product, which significantly mitigates the risk of counterfeit drugs entering the market. The increase in fake medications has resulted in heightened oversight, making efficient tracking solutions crucial for protecting public health. As noted by Rahul Gotadki, the rise in counterfeiting and falsified medicines further fuels the need for secure and efficient serialization pharma tracking systems.
- Regulatory Compliance: Following product identification mandates assists businesses in evading legal repercussions and guarantees ongoing market access. In the U.S., 90% of dispensers have implemented serialization pharma tracking capabilities to comply with the DSCSA, underscoring the widespread adoption of this system as a regulatory necessity.
- Improved Inventory Management: Serialization enhances visibility into inventory levels, enabling companies to manage stock more effectively and reduce waste. The typical expense per pack for unique identification was reported at 4.1 cents, demonstrating the financial consequences of unique identification in inventory management. This improved oversight is crucial for optimizing supply chain operations in serialization pharma and minimizing costs associated with excess inventory.
- Increased consumer trust is fostered by serialization pharma, as transparency in product tracking allows customers to verify the authenticity of the products they purchase. This trust is essential in a market increasingly concerned with safety and quality, particularly in the wake of rising counterfeit incidents.
- Operational Efficiency: Streamlined tracking processes can lead to reduced lead times and enhanced overall supply chain efficiency. Companies that have implemented advanced tracking technologies, including serialization pharma, report significant improvements in operational performance, with some observing a reduction in Overall Equipment Effectiveness (OEE) losses after implementation.
Real-world examples demonstrate these advantages: Brazil's pharmaceutical tracking expansion is fueled by government initiatives that encourage transparency and sophisticated monitoring technologies, establishing the nation as a key center for pharmaceutical production in Latin America. Additionally, partnerships like that of Arvato Systems and Advanco aim to enhance compliance and efficiency in serialization pharma, showcasing the industry's proactive approach to improving traceability and inventory management.
Conclusion
Mastering serialization in the pharmaceutical industry transcends mere regulatory compliance; it is a pivotal step towards enhancing product integrity and ensuring consumer safety. The assignment of unique identifiers to each drug unit is essential for effective tracking and tracing throughout the supply chain. This practice not only ensures adherence to evolving regulations but also plays a vital role in safeguarding public health.
Key insights from this tutorial underscore the critical role of serialization in combatting counterfeit medications, optimizing inventory management, and fostering consumer trust. By aligning with regulatory mandates such as the DSCSA and the EU FMD, pharmaceutical companies can effectively mitigate risks associated with counterfeit drugs while simultaneously enhancing operational efficiency. A structured approach to implementing serialization—ranging from assessing current systems to continuous monitoring—equips organizations with the necessary tools to navigate the complexities of compliance and operational excellence.
As the pharmaceutical landscape evolves, embracing serialization becomes essential for maintaining a competitive edge and ensuring patient safety. Companies must prioritize the integration of robust serialization systems, not only to meet regulatory requirements but also to contribute to a more transparent and secure pharmaceutical supply chain. By taking proactive steps in mastering serialization, organizations will ultimately bolster their market presence and enhance consumer confidence in the products they offer.
Frequently Asked Questions
What is serialization in pharmaceuticals?
Serialization in pharmaceuticals is the practice of assigning a unique serial number to each marketable unit of a drug, which is essential for tracking and tracing items throughout the supply chain.
Why is serialization important in the pharmaceutical industry?
Serialization is important for regulatory compliance, consumer safety, and enhancing supply chain visibility. It helps in tracking drugs from manufacturing to the end consumer, ensuring that vital information is available.
What information is typically included in the serial number?
The serial number typically includes the item's origin, batch number, and expiration date, which are crucial for regulatory compliance and consumer safety.
How is the pharmaceutical tracking market expected to grow?
The worldwide pharmaceutical tracking market is projected to expand from USD 15.1 billion in 2022 to USD 24.8 billion by 2031, with a compound annual growth rate (CAGR) of 5.1% from 2023 to 2031.
What initiative is Indonesia taking regarding serialization?
Indonesia is leading the ASEAN region by mandating item tracking for all prescription products by 2025, making it the first member state to enforce such a requirement, which enhances traceability through barcodes.
How does serialization help combat counterfeit medications?
Serialization enables each item to be verified against a secure database for authenticity, which is crucial in combating counterfeit medications, especially as AI-powered counterfeit schemes become more prevalent.
What recent changes have been introduced by the FDA regarding tracking requirements?
The FDA has introduced a stabilization phase for new tracking requirements from November 2023 to November 2024, highlighting the evolving regulatory environment in the pharmaceutical industry.
What are the benefits of implementing serialization for pharmaceutical companies?
By implementing serialization, pharmaceutical companies comply with stringent regulations, enhance supply chain visibility, improve operational efficiency, and ultimately safeguard public health.




