Overview
The article delves into the benefits, challenges, and compliance aspects of track and trace systems within the pharmaceutical industry. These systems significantly enhance patient safety and supply chain visibility through serialization. However, they also present challenges, including:
- High implementation costs
- Stringent regulatory compliance
These factors are essential for maintaining the integrity of the pharmaceutical supply chain. Addressing these challenges is critical for stakeholders aiming to ensure reliability and efficiency in their operations.
Introduction
The pharmaceutical industry is experiencing a transformative shift as it confronts the dual challenges of ensuring product safety and combating the pervasive threat of counterfeiting. Track and trace systems have emerged as essential tools in this landscape, promising enhanced visibility and security throughout the supply chain. While these systems offer significant benefits—improved patient safety and compliance with stringent regulations—they also introduce complexities and costs that can hinder widespread adoption. As stakeholders navigate this intricate terrain, the pressing question arises: how can the pharmaceutical sector effectively implement these solutions to safeguard public health while overcoming the associated challenges?
Define Track and Trace Systems in Pharmaceuticals
Track and trace system pharmaceutical represents the essential processes and technologies employed to oversee and document the journey of pharmaceutical products throughout the supply chain. These frameworks empower stakeholders to monitor the flow of drugs from production to distribution and ultimately to the end consumer. By utilizing unique identifiers, such as barcodes or RFID tags, monitoring and tracing systems ensure that each item can be accurately recognized, and its history can be traced. This capability is crucial for safeguarding product safety, combating counterfeiting, and adhering to regulatory requirements, such as the Drug Quality and Security Act, which mandates that manufacturers incorporate product identifiers on drug packages.
The global market for a track and trace system pharmaceutical is projected to reach $2.5 billion by 2025, exhibiting a compound annual growth rate (CAGR) of 12% from 2025 to 2033. This growth is driven by escalating regulatory pressures and the increasing prevalence of counterfeiting, highlighting the urgent need for serialization within the supply chain. Nonetheless, challenges such as significant initial investment costs and the complexity of system integration may impede the widespread adoption of these solutions.
Furthermore, advancements in technologies like blockchain and artificial intelligence are being explored to bolster security and transparency in the pharmaceutical supply chain. Ensuring data accuracy is paramount for the reliability of monitoring and tracing data, facilitating effective implementation. As the market evolves, it is imperative for stakeholders to navigate these challenges while leveraging the benefits of a track and trace system pharmaceutical to protect consumers and reinforce trust in the healthcare framework.
Explain the Role of Serialization in Track and Trace
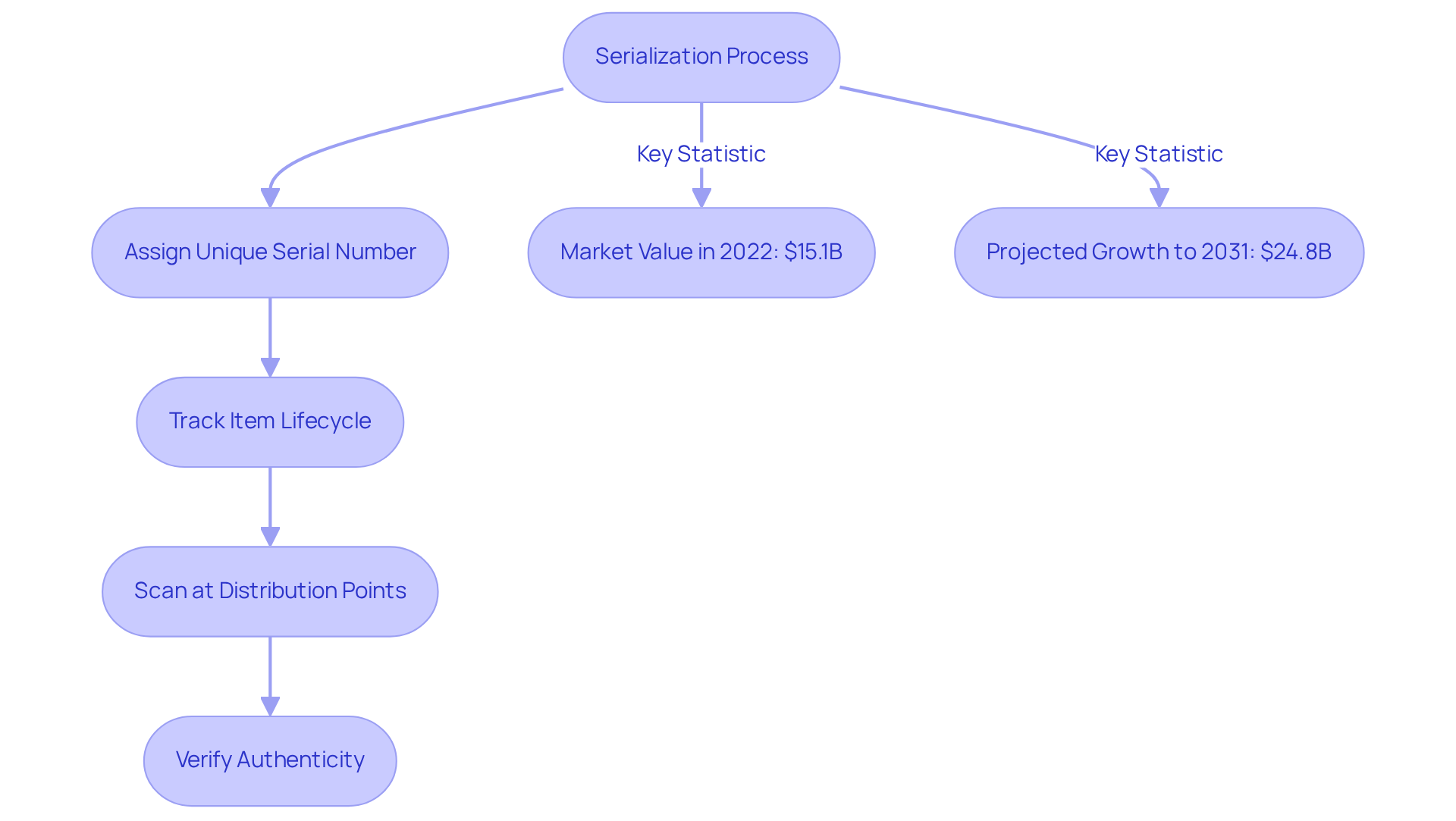
Serialization assigns a unique serial number to each saleable unit of a pharmaceutical item, which is fundamental to the track and trace system pharmaceutical. This unique identifier is essential for accurately tracking each item throughout its lifecycle. By enabling precise tracking, serialization significantly contributes to the prevention of counterfeit drugs from infiltrating the supply chain. Scanning serialized codes at various distribution points enables stakeholders to verify authenticity and ensure adherence to stringent regulatory standards.
The global pharmaceutical serialization market, valued at approximately 15.1 billion USD in 2022, is projected to grow at a compound annual growth rate (CAGR) of 5.1%, reaching 24.8 billion USD by 2031. This growth is driven by increasing regulatory pressures and the rising demand for . Significantly, the incorporation of advanced technologies such as RFID and 2D barcodes is improving traceability and compliance, further reinforcing the role of serialization in protecting public health.
Experts in the field emphasize that effective serialization not only enhances drug safety but also improves supply chain visibility. For instance, Roche has made strategic investments in serialization technologies to ensure robust verification of items and compliance with evolving regulations. These investments reflect broader industry trends towards enhancing operational capabilities and meeting regulatory demands. As the drug industry continues to adjust to these challenges, the adoption of serialization methods is becoming more essential for preserving medication authenticity and safeguarding consumers from counterfeit items. Moreover, the average expense per pack for serialization is roughly 4.1 cents, emphasizing the financial consequences of adopting these solutions. The complexities of the drug supply chain further emphasize the necessity of robust serialization practices.
Discuss Benefits of Track and Trace Systems
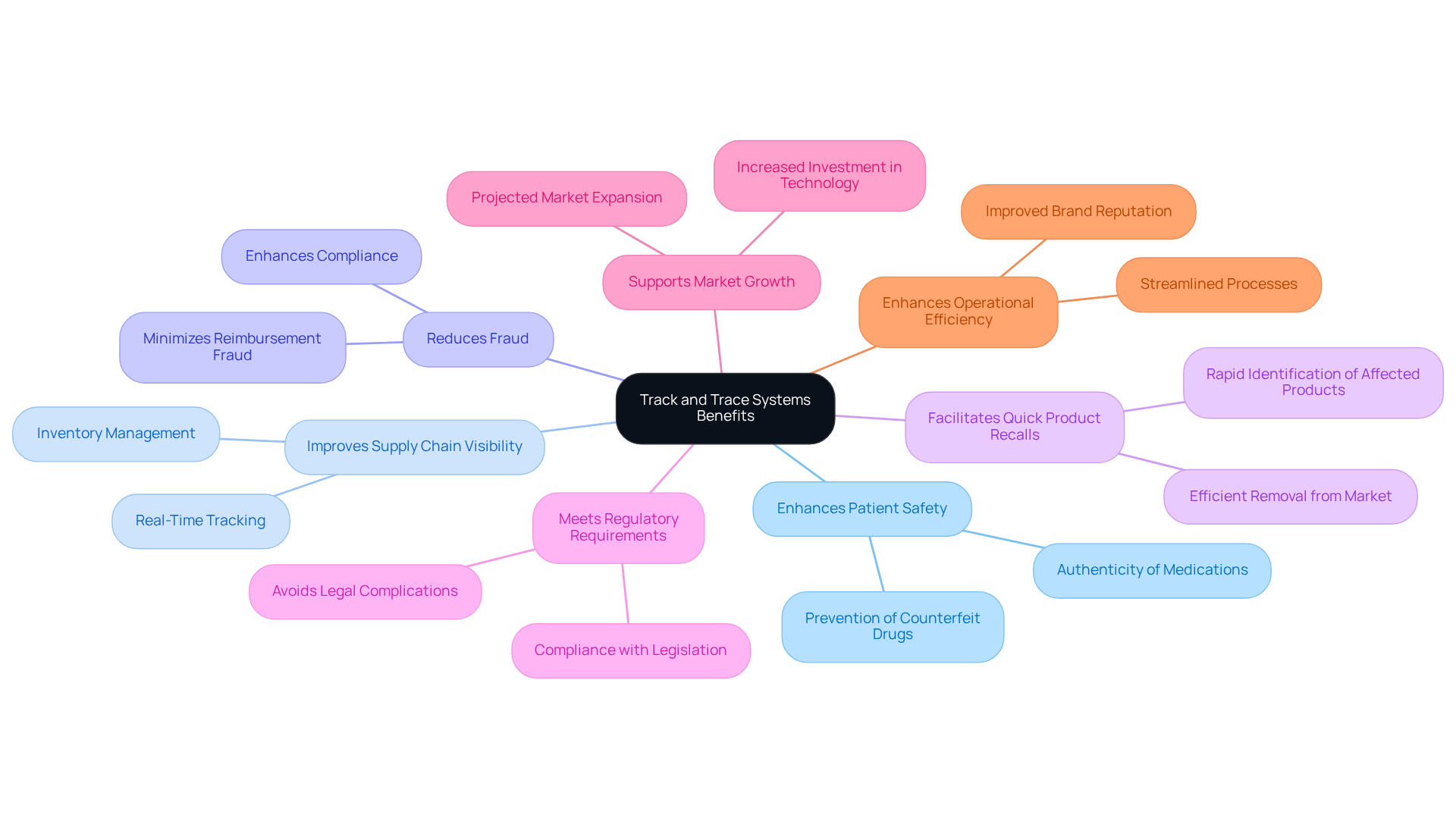
The advantages of monitoring and tracing methods in the drug industry, particularly the pharmaceutical, are numerous and substantial.
Firstly, they significantly enhance patient safety by utilizing a track and trace system pharmaceutical to ensure that only authentic medications reach consumers.
Secondly, these frameworks enhance supply chain visibility by implementing a track and trace system pharmaceutical, which facilitates better inventory management and reduces the risk of stockouts.
For instance, Turkey's successful implementation of a comprehensive track and trace system pharmaceutical has set a benchmark, substantially minimizing reimbursement fraud and bolstering supply chain integrity.
Moreover, the track and trace system pharmaceutical allows for quicker and more effective product recalls in the event of safety concerns, thereby protecting public health.
Furthermore, they assist drug companies in meeting regulatory requirements through a track and trace system pharmaceutical, thereby lowering the risk of penalties and legal complications.
As the global market for monitoring and tracing solutions is projected to reach 4.8 billion U.S. dollars by 2029, understanding its historical growth from 2019 to 2023 is essential for recognizing the increasing focus on compliance.
Overall, the implementation of a track and trace system pharmaceutical enhances operational efficiency and strengthens brand reputation, underscoring a commitment to quality and safety in the medication supply chain.
Identify Challenges in Implementing Track and Trace Solutions
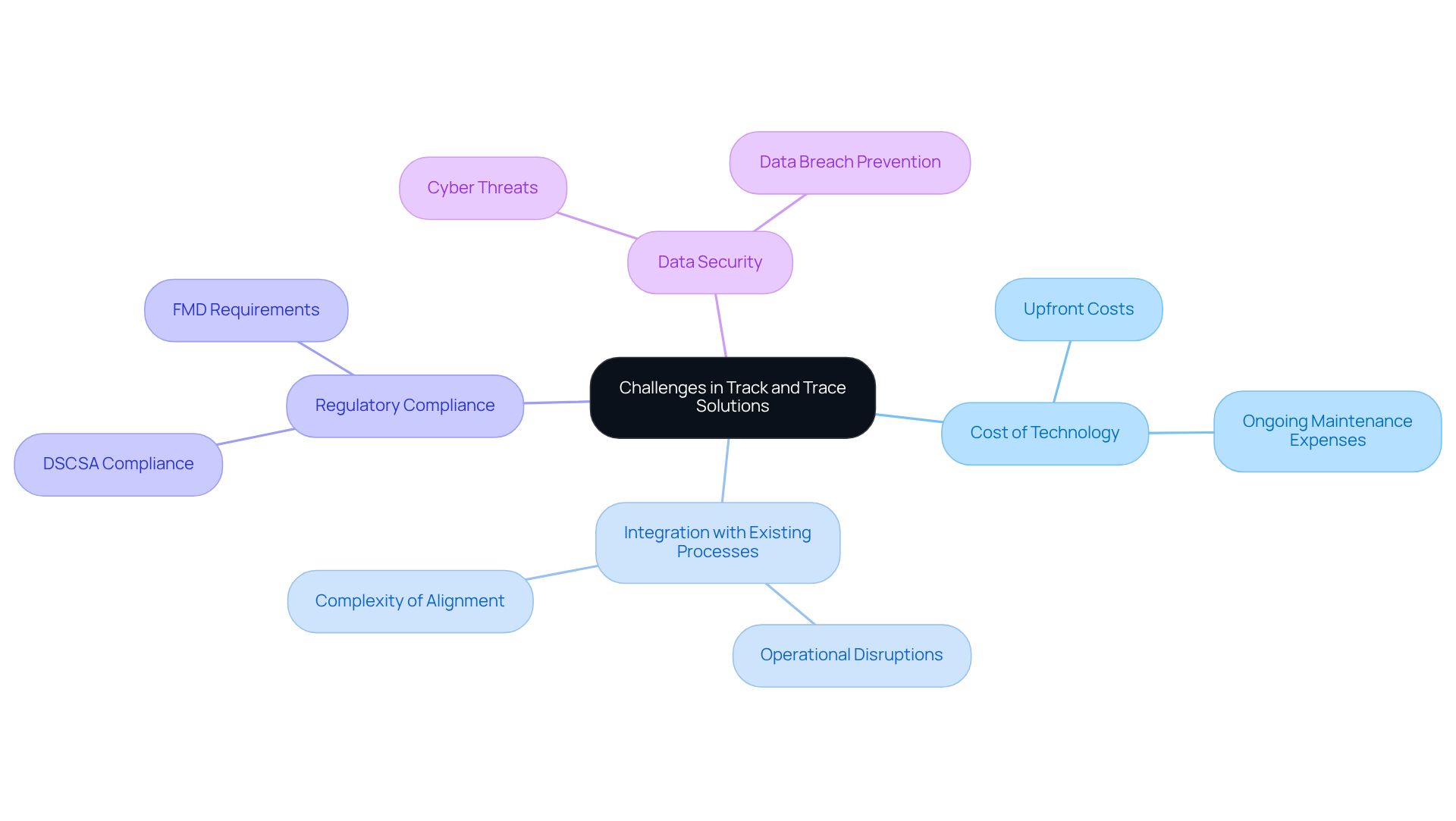
Implementing a track and trace system pharmaceutical in the industry presents several significant challenges. A primary concern is the substantial cost associated with technology and infrastructure upgrades necessary for effective serialization and tracking. The initial investment in can be a barrier, particularly for small and medium-sized enterprises (SMEs), which often struggle with the upfront costs and ongoing maintenance expenses. As noted by industry experts, these financial implications can hinder the adoption of advanced tracking technologies.
Moreover, companies frequently encounter difficulties when integrating new systems with existing processes, leading to operational disruptions. The complexity of aligning tracing solutions with current workflows necessitates careful planning and execution to minimize interruptions.
Regulatory compliance adds another layer of complexity, as pharmaceutical companies must navigate varying requirements across different regions. For example, the U.S. Drug Supply Chain Security Act (DSCSA) mandates serialization for prescription drugs, while the European Union's Falsified Medicines Directive (FMD) imposes strict serialization standards. Failure to comply with these regulations can result in significant penalties and loss of market access.
Furthermore, ensuring data security is essential, as monitoring and tracing mechanisms manage sensitive information that is susceptible to cyber threats. Companies must implement strong security measures to safeguard against data breaches and uphold the integrity of their tracking processes. Tackling these obstacles is crucial for the effective deployment of a track and trace system pharmaceutical, which allows drug companies to improve product safety and efficiently combat counterfeiting.
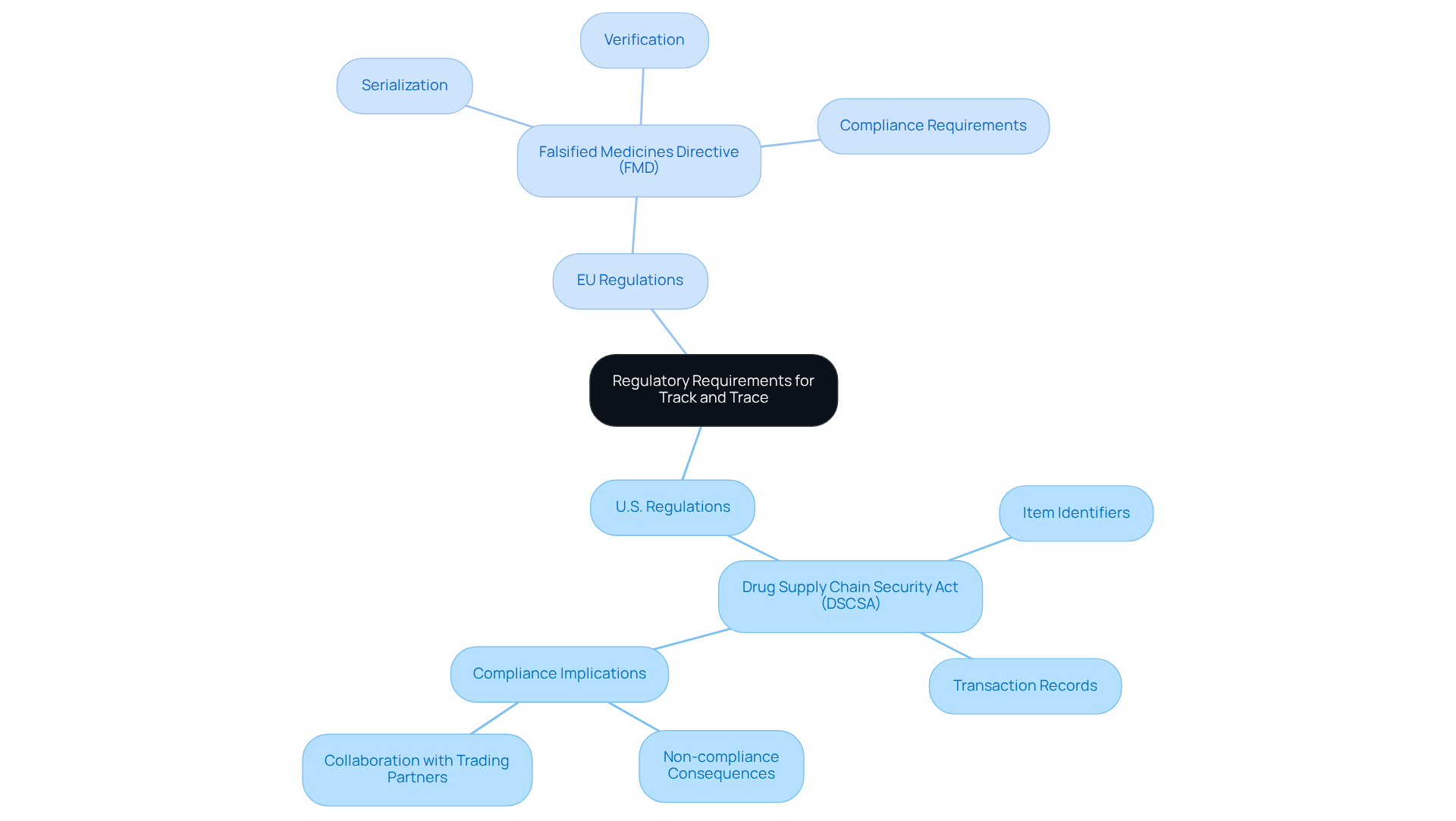
Review Regulatory Requirements for Track and Trace
Regulatory requirements for monitoring and tracing in the drug industry are fundamentally influenced by laws aimed at improving drug safety and combating counterfeiting. In the United States, the Drug Supply Chain Security Act (DSCSA) mandates that pharmaceutical companies establish robust track and trace systems to monitor prescription drugs throughout the supply chain. This encompasses the implementation of distinctive item identifiers and the maintenance of detailed transaction records. Similarly, the European Union's Falsified Medicines Directive (FMD) requires serialization and verification of medicinal items, ensuring that each one can be traced back to its origin.
Adhering to these regulations necessitates meticulous record-keeping of item movements, secure data management practices, and the preparedness of all stakeholders in the supply chain to meet these stringent standards. Currently, over 20,000 prescription drug items are approved for marketing in the U.S., with the final enforcement deadline for the DSCSA set for November 27, 2024. Non-compliance can lead to severe repercussions, including regulatory fines, loss of business opportunities, and potential legal liabilities, significantly impacting a company's market access and reputation.
Statistics reveal that approximately 39% of companies utilize a track and trace system for demand forecasting, which can lead to a reduction in inventory levels by 20-30%. This highlights the dual benefit of compliance: not only does it ensure adherence to regulations, but it also . Experts emphasize that collaboration with trading partners is essential for smooth compliance with DSCSA requirements, as the interconnected nature of the supply chain demands a unified approach to data sharing and verification. Failure to comply with these regulations can expose manufacturers to serious risks, including product recalls and reputational damage, underscoring the critical importance of adhering to the DSCSA and FMD standards.
Conclusion
The implementation of track and trace systems in the pharmaceutical industry is crucial for ensuring the safety and integrity of medications throughout the supply chain. These systems not only enhance product visibility and combat counterfeiting but also facilitate compliance with stringent regulatory requirements, ultimately reinforcing consumer trust in the healthcare system.
Key insights explored throughout the article highlight:
- The critical role of serialization in enhancing drug safety.
- The numerous benefits of track and trace systems—including improved inventory management and expedited product recalls.
- The challenges companies face in adopting these technologies.
Regulatory requirements, such as the Drug Supply Chain Security Act and the European Union's Falsified Medicines Directive, further underscore the necessity for robust tracking mechanisms to safeguard public health.
As the pharmaceutical landscape continues to evolve, embracing track and trace systems will be essential for companies aiming to navigate the complexities of compliance and operational efficiency. Stakeholders are encouraged to invest in these technologies, collaborate with partners, and prioritize the adoption of best practices. This approach not only meets regulatory demands but also enhances overall supply chain resilience and consumer confidence. The future of pharmaceutical safety hinges on the commitment to transparency and accountability that these systems provide.
Frequently Asked Questions
What are track and trace systems in pharmaceuticals?
Track and trace systems in pharmaceuticals are processes and technologies that monitor and document the journey of pharmaceutical products throughout the supply chain, ensuring that stakeholders can track the flow of drugs from production to distribution and to the end consumer.
How do track and trace systems ensure product safety?
These systems utilize unique identifiers, such as barcodes or RFID tags, which allow each item to be accurately recognized and its history traced. This capability is crucial for safeguarding product safety, combating counterfeiting, and adhering to regulatory requirements.
What is the projected market growth for track and trace systems in pharmaceuticals?
The global market for track and trace systems in pharmaceuticals is projected to reach $2.5 billion by 2025, with a compound annual growth rate (CAGR) of 12% from 2025 to 2033.
What challenges do track and trace systems face?
Challenges include significant initial investment costs and the complexity of system integration, which may impede the widespread adoption of these solutions.
What role does serialization play in track and trace systems?
Serialization assigns a unique serial number to each saleable unit of a pharmaceutical item, which is essential for accurately tracking each item throughout its lifecycle and preventing counterfeit drugs from entering the supply chain.
How is the pharmaceutical serialization market expected to grow?
The global pharmaceutical serialization market was valued at approximately 15.1 billion USD in 2022 and is projected to grow at a CAGR of 5.1%, reaching 24.8 billion USD by 2031.
What technologies are improving traceability and compliance in serialization?
Advanced technologies such as RFID and 2D barcodes are being incorporated to enhance traceability and compliance within the serialization process.
What are the financial implications of implementing serialization?
The average expense per pack for serialization is roughly 4.1 cents, highlighting the financial consequences of adopting these solutions in the pharmaceutical industry.
Why is data accuracy important in track and trace systems?
Ensuring data accuracy is paramount for the reliability of monitoring and tracing data, which facilitates effective implementation and reinforces trust in the healthcare framework.




